Question: please finish this with all the steps, the same question on chegg was wrong. One kmol of liquid propylene is contained in a sealed vessel
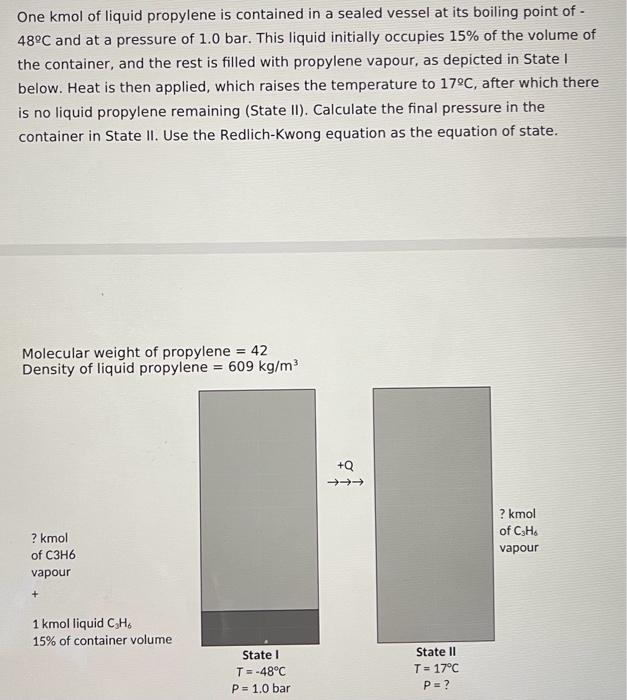
One kmol of liquid propylene is contained in a sealed vessel at its boiling point of 48C and at a pressure of 1.0bar. This liquid initially occupies 15% of the volume of the container, and the rest is filled with propylene vapour, as depicted in State I below. Heat is then applied, which raises the temperature to 17C, after which there is no liquid propylene remaining (State II). Calculate the final pressure in the container in State II. Use the Redlich-Kwong equation as the equation of state. Molecular weight of propylene =42 Density of liquid propylene =609kg/m3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


