Question: please finish this with all the steps This problem concerns a mixture of benzene (1) and acetonitrile (2) in which the vapour-phase is an ideal-gas
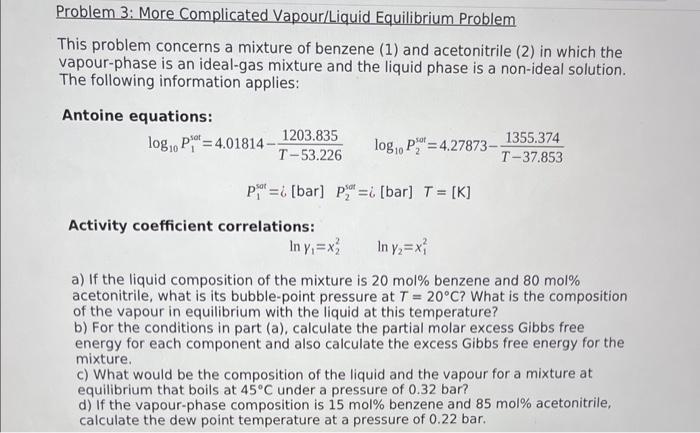
This problem concerns a mixture of benzene (1) and acetonitrile (2) in which the vapour-phase is an ideal-gas mixture and the liquid phase is a non-ideal solution. The following information applies: Antoine equations: log10P1sot=4.01814T53.2261203.835log10P2sot=4.27873T37.8531355.374P1sot=i[barP2sot=i[bar]=[K] Activity coefficient correlations: ln1=x22ln2=x12 a) If the liquid composition of the mixture is 20mol% benzene and 80mol% acetonitrile, what is its bubble-point pressure at T=20C ? What is the composition of the vapour in equilibrium with the liquid at this temperature? b) For the conditions in part (a), calculate the partial molar excess Gibbs free energy for each component and also calculate the excess Gibbs free energy for the mixture. c) What would be the composition of the liquid and the vapour for a mixture at equilibrium that boils at 45C under a pressure of 0.32 bar? d) If the vapour-phase composition is 15mol% benzene and 85mol acetonitrile, calculate the dew point temperature at a pressure of 0.22bar. This problem concerns a mixture of benzene (1) and acetonitrile (2) in which the vapour-phase is an ideal-gas mixture and the liquid phase is a non-ideal solution. The following information applies: Antoine equations: log10P1sot=4.01814T53.2261203.835log10P2sot=4.27873T37.8531355.374P1sot=i[barP2sot=i[bar]=[K] Activity coefficient correlations: ln1=x22ln2=x12 a) If the liquid composition of the mixture is 20mol% benzene and 80mol% acetonitrile, what is its bubble-point pressure at T=20C ? What is the composition of the vapour in equilibrium with the liquid at this temperature? b) For the conditions in part (a), calculate the partial molar excess Gibbs free energy for each component and also calculate the excess Gibbs free energy for the mixture. c) What would be the composition of the liquid and the vapour for a mixture at equilibrium that boils at 45C under a pressure of 0.32 bar? d) If the vapour-phase composition is 15mol% benzene and 85mol acetonitrile, calculate the dew point temperature at a pressure of 0.22bar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


