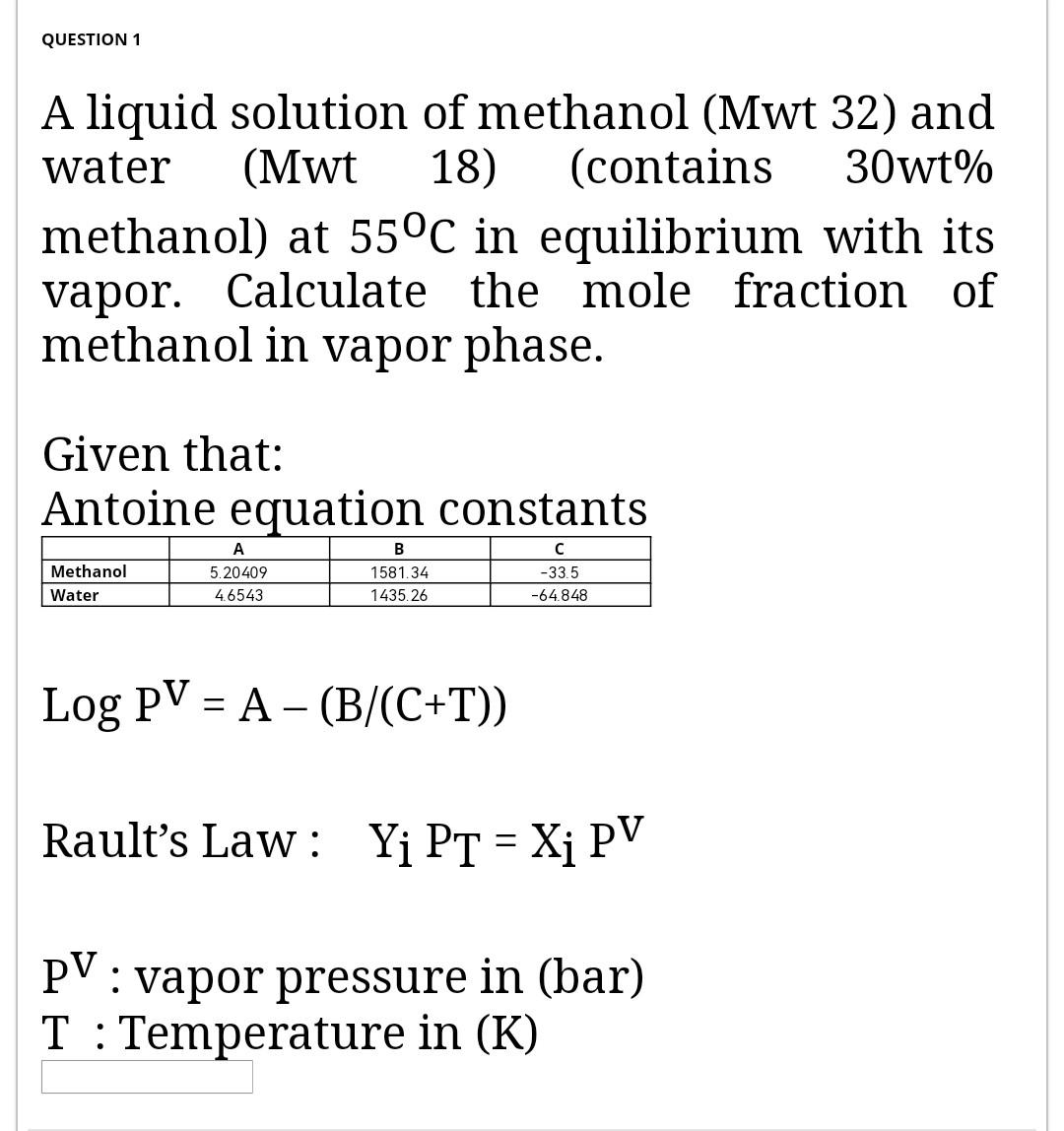
Question: please focus I need right answer QUESTION 1 A liquid solution of methanol (Mwt 32) and water (Mwt 18) (contains 30wt% methanol) at 55C in
please focus I need right answer
QUESTION 1 A liquid solution of methanol (Mwt 32) and water (Mwt 18) (contains 30wt% methanol) at 55C in equilibrium with its vapor. Calculate the mole fraction of methanol in vapor phase. Given that: Antoine equation constants Methanol Water 5.20409 4.6543 B 1581.34 1435.26 -33.5 -64.848 Log PV = A - (B/(C+T)) Rault's Law: Y PT = Xi PV = PV: vapor pressure in (bar) T : Temperature in (K)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


