Question: Please give me a detailed solution and answer to this problem. STOICHIOMETRIC RATIOS. Aside from the decomposition of organic carbon compounds such as glucose, other
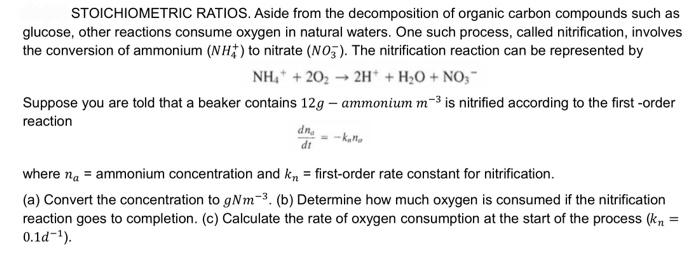
STOICHIOMETRIC RATIOS. Aside from the decomposition of organic carbon compounds such as glucose, other reactions consume oxygen in natural waters. One such process, called nitrification, involves the conversion of ammonium (NH4+)to nitrate (NO3). The nitrification reaction can be represented by NH4++2O22H++H2O+NO3 Suppose you are told that a beaker contains 12g ammonium m3 is nitrified according to the first -order reaction dtdnu=knno where na= ammonium concentration and kn= first-order rate constant for nitrification. (a) Convert the concentration to gNm3. (b) Determine how much oxygen is consumed if the nitrification reaction goes to completion. (c) Calculate the rate of oxygen consumption at the start of the process (kn= 0.1d1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


