Question: PLEASE GIVE ME CORRECT ANSWER I WILL THUMBS UP I PROMISE IF YOU GIVE ME CORRECT ANSWER THANK YOU IN ADVANCE Consider the following reaction,
PLEASE GIVE ME CORRECT ANSWER I WILL THUMBS UP I PROMISE IF YOU GIVE ME CORRECT ANSWER THANK YOU IN ADVANCE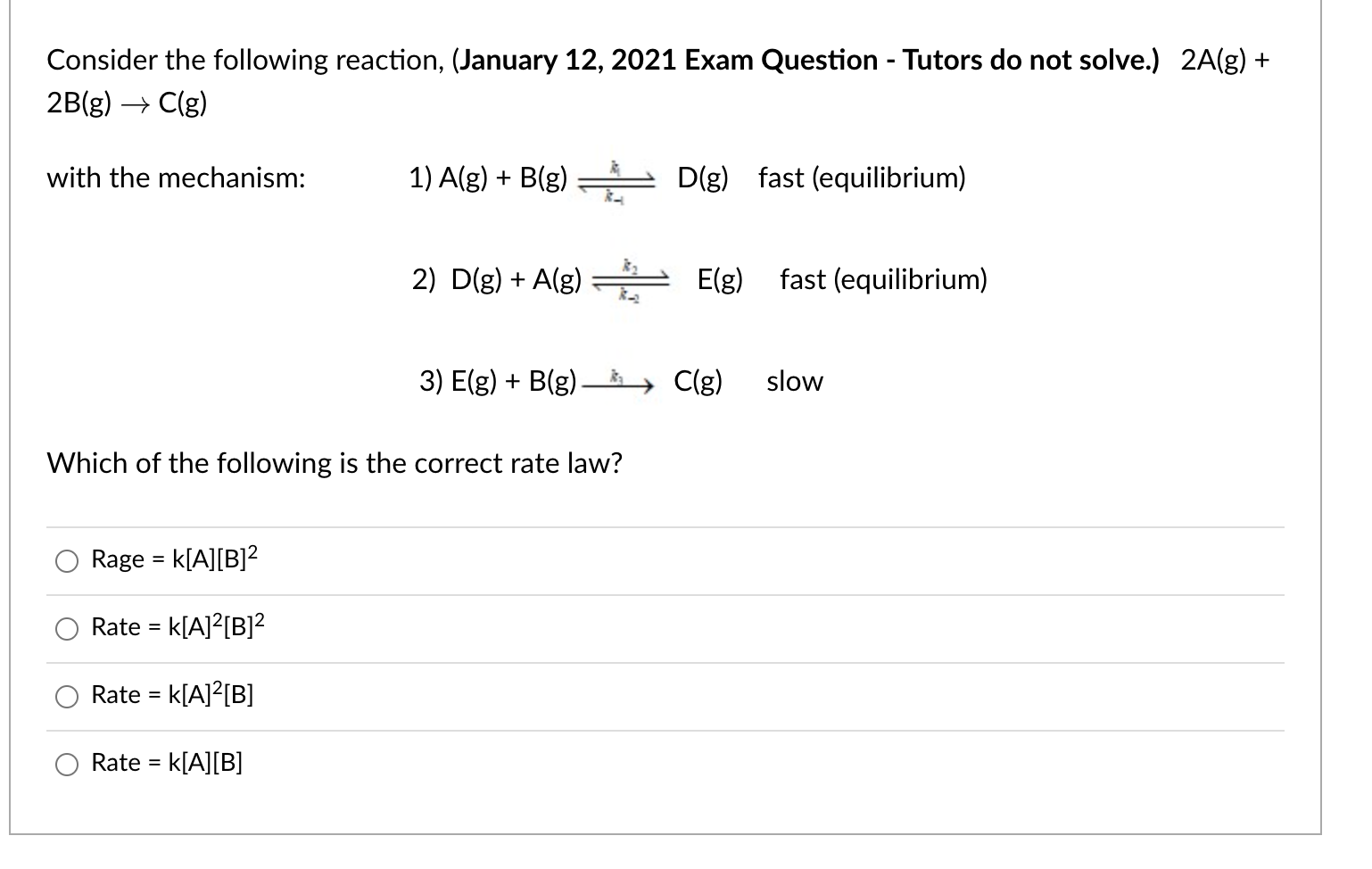
Consider the following reaction, (January 12, 2021 Exam Question - Tutors do not solve.) 2A(g) + 2B(g) C(g) with the mechanism: 1) A(g) + B(g) D(g) fast (equilibrium) 2) D(g) +A(g) E(g) fast (equilibrium) 3) E(g) + B(g) Clg) slow Which of the following is the correct rate law? Rage = k[A][B]2 Rate = k[A]2[B]2 Rate = k[A]?[B] Rate = k[A][B]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


