Question: Please giver concise explanation with formulas and calculations with ICE Table if needed For the solution of 0.025M lactic acid, HC3H5O2(Ka=8.4104), calculate: a) [H3O+]b)pH
Please giver concise explanation with formulas and calculations with ICE Table if needed
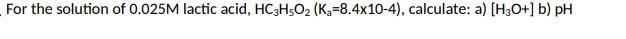
For the solution of 0.025M lactic acid, HC3H5O2(Ka=8.4104), calculate: a) [H3O+]b)pH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


