Question: please hellllp Use the integrated rate law to calculate time for percent reacted. The decomposition of nitramide in aqueous solution at 25C is first order
please hellllp 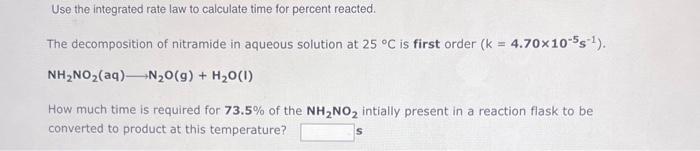

Use the integrated rate law to calculate time for percent reacted. The decomposition of nitramide in aqueous solution at 25C is first order (k=4.70105s1). NH2NO2(aq)N2O(g)+H2O(I) How much time is required for 73.5% of the NH2NO2 intially present in a reaction flask to be converted to product at this temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


