Question: Please Help 1. 2. ANSWER IN 3 SIGNIFICANT FIGURES Consider the data below for the reaction X products. Note: You will have to determine the
Please Help
1.
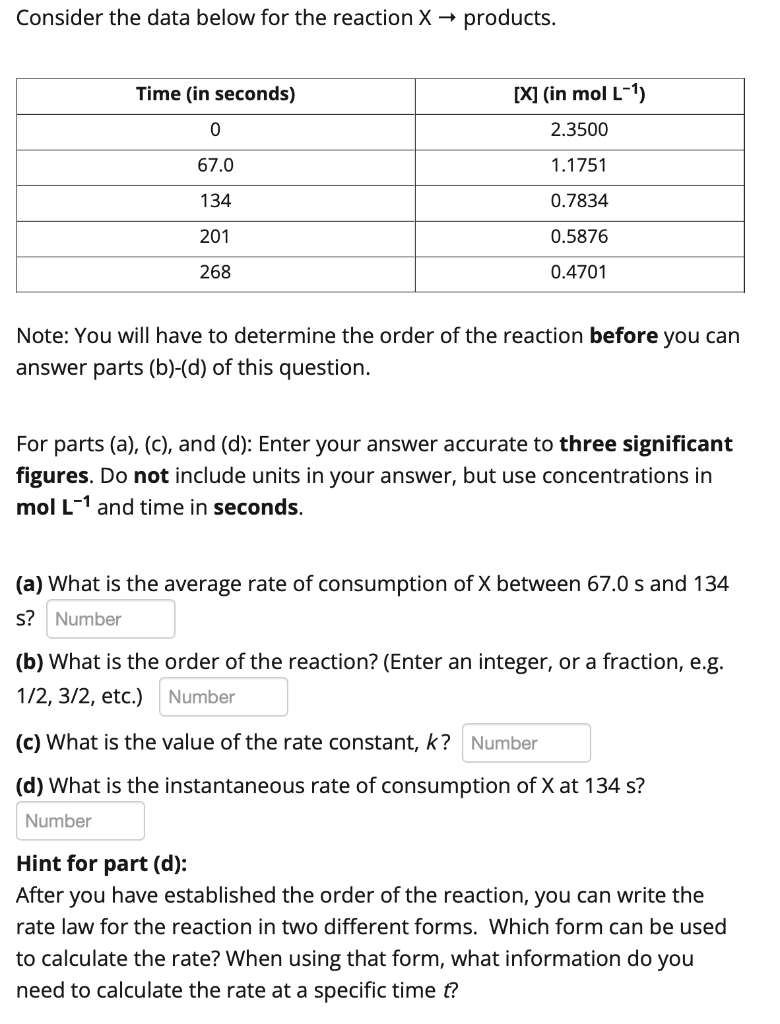
2. ANSWER IN 3 SIGNIFICANT FIGURES
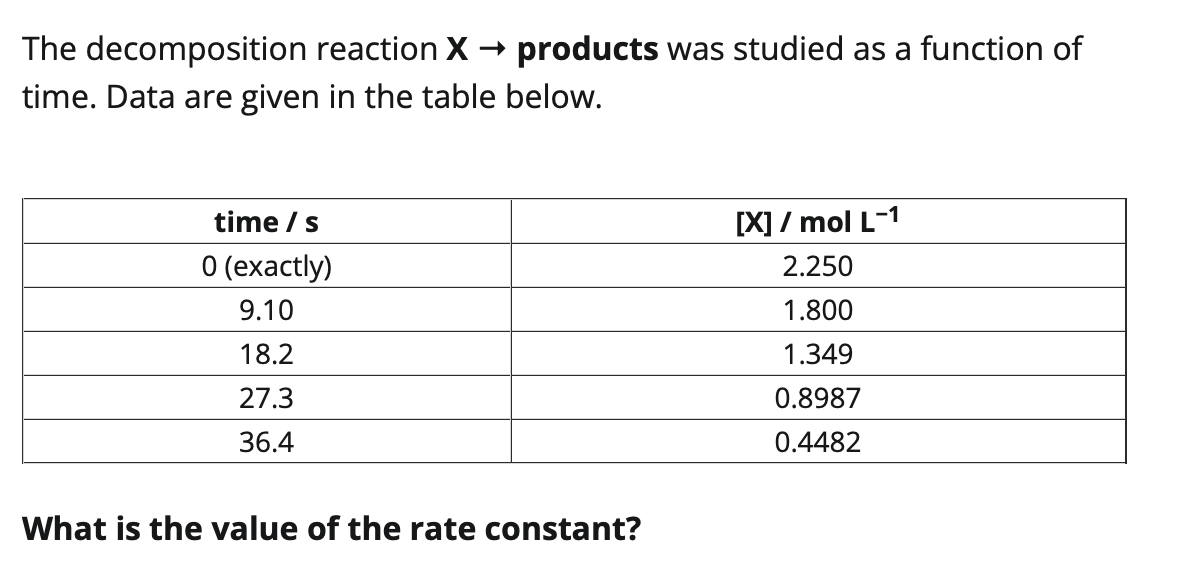
Consider the data below for the reaction X products. Note: You will have to determine the order of the reaction before you can answer parts (b)-(d) of this question. For parts (a), (c), and (d): Enter your answer accurate to three significant figures. Do not include units in your answer, but use concentrations in mol L1 and time in seconds. (a) What is the average rate of consumption of X between 67.0s and 134 s? (b) What is the order of the reaction? (Enter an integer, or a fraction, e.g. 1/2,3/2, etc.) (c) What is the value of the rate constant, k ? (d) What is the instantaneous rate of consumption of X at 134s ? Hint for part (d): After you have established the order of the reaction, you can write the rate law for the reaction in two different forms. Which form can be used to calculate the rate? When using that form, what information do you need to calculate the rate at a specific time t ? The decomposition reaction X products was studied as a function of time. Data are given in the table below. What is the value of the rate constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


