Question: please help 1. Lewis Structure (write over the dashed lines with solid lines showing single, double, or triple bonds, as appropriate): Total # of valence
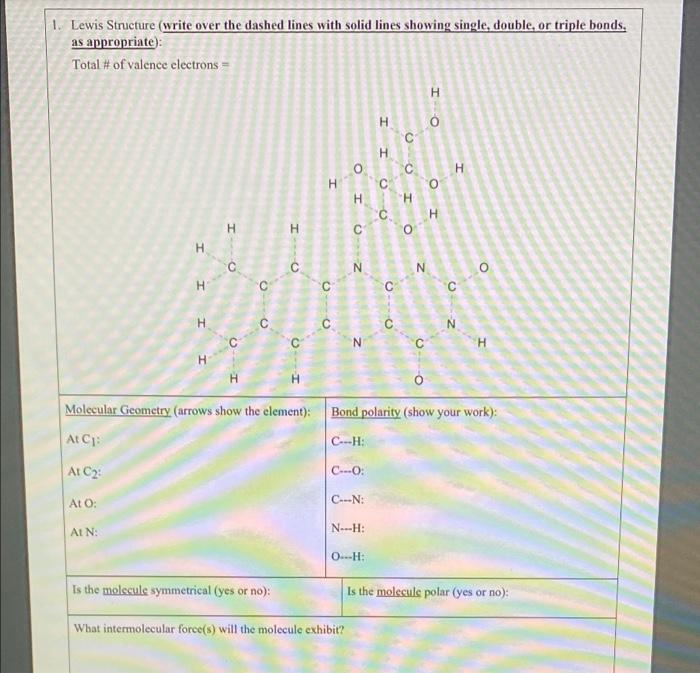
1. Lewis Structure (write over the dashed lines with solid lines showing single, double, or triple bonds, as appropriate): Total # of valence electrons = H H I H o H H O H H H H H H C N N H C C H H H 0 Molecular Geometry (arrows show the element): Bond polarity (show your work): At CH: At C: C...O At O: C. N: AIN: NH: OH: Is the molecule symmetrical (yes or no): Is the moleculs polar (yes or no): What intermolecular force(s) will the molecule exhibit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


