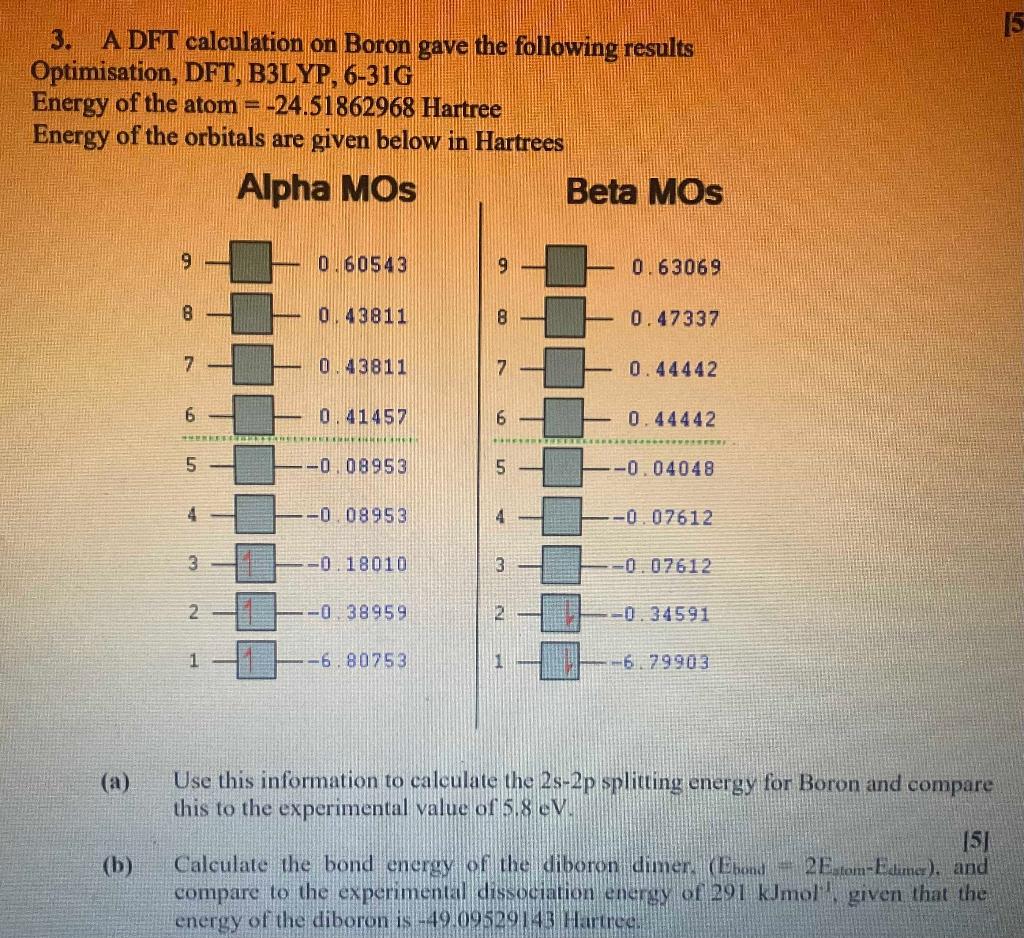
Question: Please help 15 3. A DFT calculation on Boron gave the following results Optimisation, DFT, B3LYP, 6-316 Energy of the atom = -24.51862968 Hartree Energy
 Please help
Please help
15 3. A DFT calculation on Boron gave the following results Optimisation, DFT, B3LYP, 6-316 Energy of the atom = -24.51862968 Hartree Energy of the orbitals are given below in Hartrees Alpha MOs Beta MOs 9 0.60543 9 0.63069 8 0.43811 8 0.47337 7 0.43811 7 0.44442 6 0.41457 6 0.44442 5 --008953 5 -0.04048 4 -0 08953 4 - -0 07612 3 -0.18010 3 -0.07612 2. - 38959 2 -0.34591 1 -6. 80753 1 -6,79903 (a) b Use this information to calculate the 2s-2p splitting energy for Boron and compare this to the experimental value of 5.8 16V [5] Calculate the bond energy of the diboron dimer. (Eron 2E tom-Edlimer), and compare to the experimental dissociation energy of 291 kJmolgiven that the energy of the diboron is -49.09529143 Hartreer (b) 15 3. A DFT calculation on Boron gave the following results Optimisation, DFT, B3LYP, 6-316 Energy of the atom = -24.51862968 Hartree Energy of the orbitals are given below in Hartrees Alpha MOs Beta MOs 9 0.60543 9 0.63069 8 0.43811 8 0.47337 7 0.43811 7 0.44442 6 0.41457 6 0.44442 5 --008953 5 -0.04048 4 -0 08953 4 - -0 07612 3 -0.18010 3 -0.07612 2. - 38959 2 -0.34591 1 -6. 80753 1 -6,79903 (a) b Use this information to calculate the 2s-2p splitting energy for Boron and compare this to the experimental value of 5.8 16V [5] Calculate the bond energy of the diboron dimer. (Eron 2E tom-Edlimer), and compare to the experimental dissociation energy of 291 kJmolgiven that the energy of the diboron is -49.09529143 Hartreer (b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


