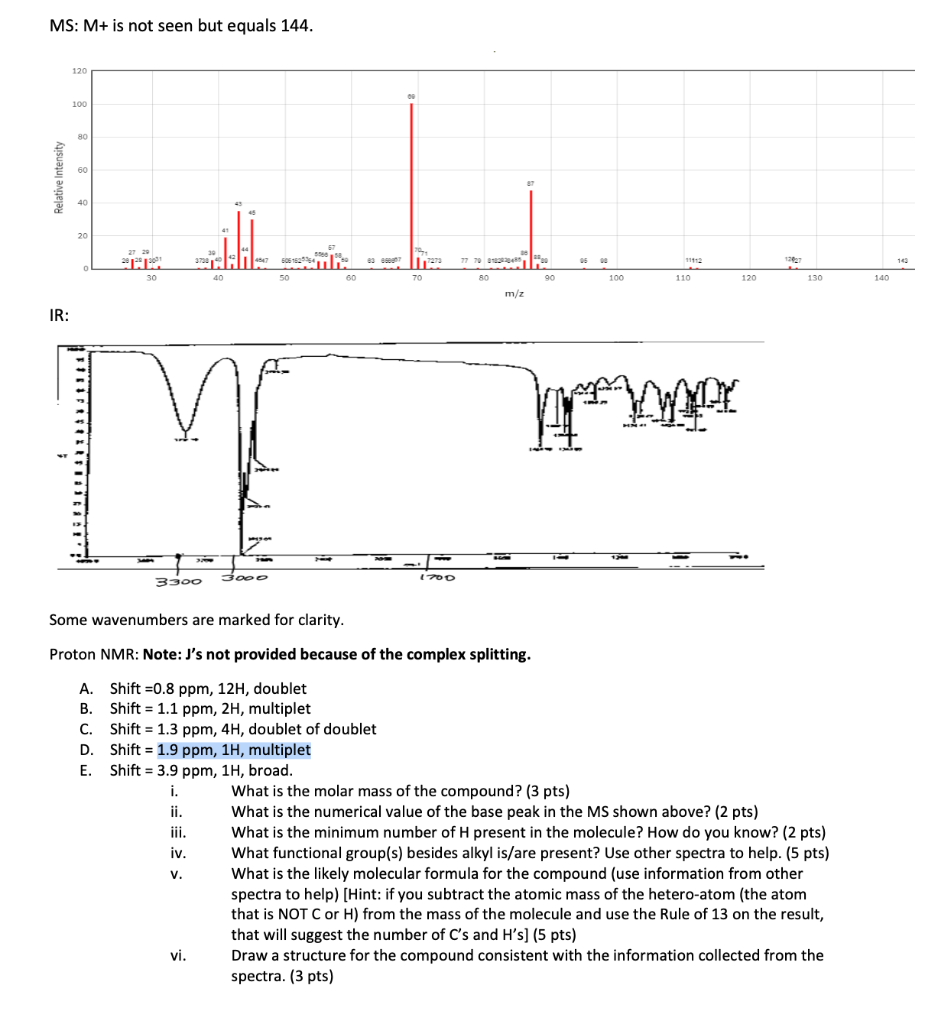
Question: please help 1-5 MS: M+ is not seen but equals 144 . IR: Some wavenumbers are marked for clarity. Proton NMR: Note: J's not provided
please help 1-5
MS: M+ is not seen but equals 144 . IR: Some wavenumbers are marked for clarity. Proton NMR: Note: J's not provided because of the complex splitting. A. Shift =0.8ppm,12H, doublet B. Shift =1.1ppm,2H, multiplet C. Shift =1.3ppm,4H, doublet of doublet D. Shift =1.9ppm,1H, multiplet E. Shift=3.9ppm,1H, broad. i. What is the molar mass of the compound? (3 pts) ii. What is the numerical value of the base peak in the MS shown above? ( 2pts) iii. What is the minimum number of H present in the molecule? How do you know? ( 2pts ) iv. What functional group(s) besides alkyl is/are present? Use other spectra to help. (5 pts) v. What is the likely molecular formula for the compound (use information from other spectra to help) [Hint: if you subtract the atomic mass of the hetero-atom (the atom that is NOT C or H) from the mass of the molecule and use the Rule of 13 on the result, that will suggest the number of Cs and Hs ( 5pts) vi. Draw a structure for the compound consistent with the information collected from the spectra. (3 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


