Question: please help 7,8 and 9 7) Rate and stoichiometry: Consider the reaction given at 150 C. H2(e)+12(g) 2H1 a) Fill the table Time in sec
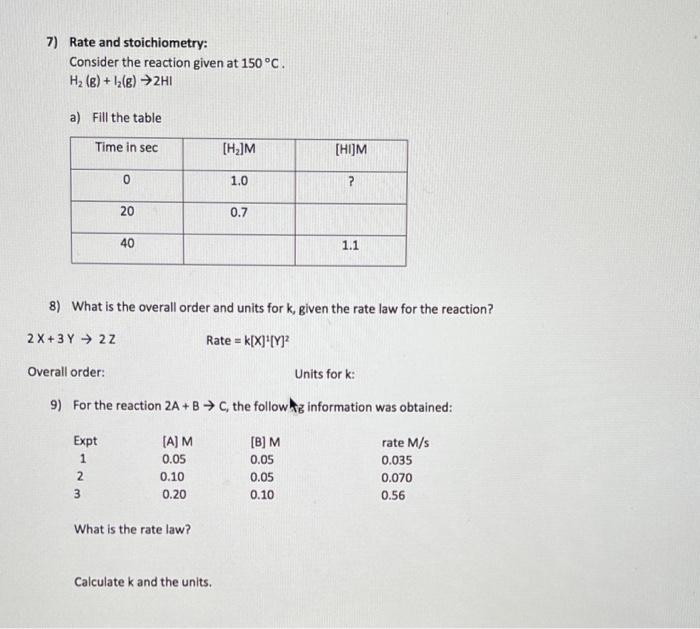
7) Rate and stoichiometry: Consider the reaction given at 150 C. H2(e)+12(g) 2H1 a) Fill the table Time in sec [H]M (HIJM 0 1.0 ? 20 0.7 40 1.1 8) What is the overall order and units for k, given the rate law for the reaction? 2 X + 3 Y 22 Rate = k[X]*[Y] Overall order: Units for k: 9) For the reaction 2A+B C, the follow us information was obtained: - Expt 1 2 3 (A)M 0.05 0.10 0.20 [B]M 0.05 0.05 0.10 rate M/S 0.035 0.070 0.56 What is the rate law? Calculate k and the units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


