Question: please help A certain liquid X has a normal boiling point of 103.10C and a boiling point elevation constant Kb=1.21Ckgmol1. Calculate the boiling point of
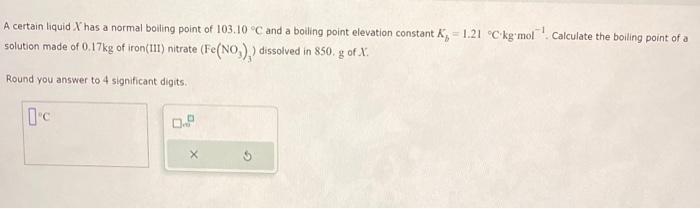
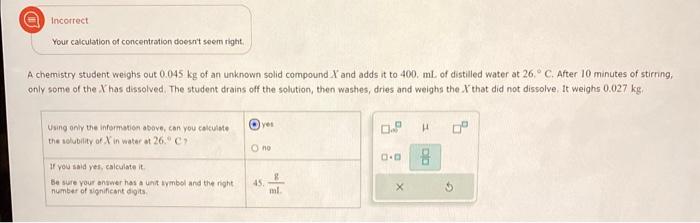
A certain liquid X has a normal boiling point of 103.10C and a boiling point elevation constant Kb=1.21Ckgmol1. Calculate the boiling point of a Round you answer to 4 significant digits. Incorrect Your calculation of concentration doesn't seem right. A chemistry student weighs out 0.045kg of an unknown solid compound X and adds it to 400,mL of distilled water at 26C. After 10 minutes of stirning; only some of the Y has dissolved. The student drains off the solution, then washes, dries and weighs the X that did not dissolve. It weighs 0.027 kg. Using onil the information above, can you cakulate the selubility of X in watar at 26C ? If you said yef, ealeulate it. be sure your anywer has a unit uymbol and the right. number of rignifieant digits. 45. mL8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


