Question: Please help!!! A mixture initially contains A,B, and C in the following concentrations: [A]=0.500M,[B]=0.650M, and [C]= 0.450M. The following reaction occurs and equilibrium is established:
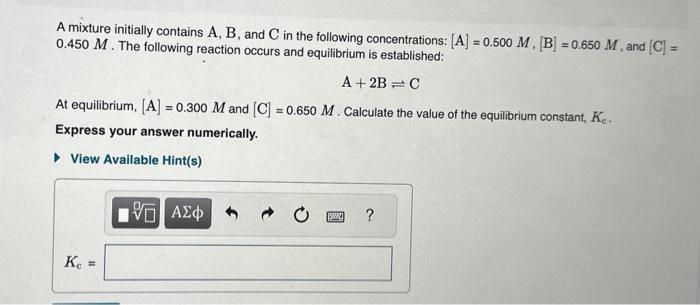
![following concentrations: [A]=0.500M,[B]=0.650M, and [C]= 0.450M. The following reaction occurs and equilibrium](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66fa69bcef750_10066fa69bc8cba0.jpg)
![is established: A+2BC At equilibrium, [A]=0.300M and [C]=0.650M. Calculate the value of](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66fa69bd8df1e_10166fa69bd295bd.jpg)
A mixture initially contains A,B, and C in the following concentrations: [A]=0.500M,[B]=0.650M, and [C]= 0.450M. The following reaction occurs and equilibrium is established: A+2BC At equilibrium, [A]=0.300M and [C]=0.650M. Calculate the value of the equilibrium constant, Kc. Express your answer numerically. The following reaction was carried out in a 4.00L reaction vessel at 1100K : C(s)+H2O(g)CO(g)+H2(g) If during the course of the reaction, the vessel is found to contain 6.25mol of C,13.4mol of H2O,3.50mol of CO , and 8.00mol of H2, what is the reaction quotient Qc ? Enter the reaction quotient numerically. A certain reaction has an activation energy of 70.0kJ/mol and a frequency factor of A1=3.601012M1s1. What is the rate constant, k, of this reaction at 29.0C ? Express your answer with the appropriate units. Indicate the multiplication of units explicitly either with a multiplication dot (asterisk) or a dash
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


