Question: Please Help!!!! A. STANDARD SAMPLE PREPARATION B. ABSORBANCE AND CONCENTRATION DATA Concentration of stock nickel sulfate hexahydrate solution Calculations: M1V1=M2V2 sample 1: (5)(0.400)=M2(50)=0.04M sample 2:
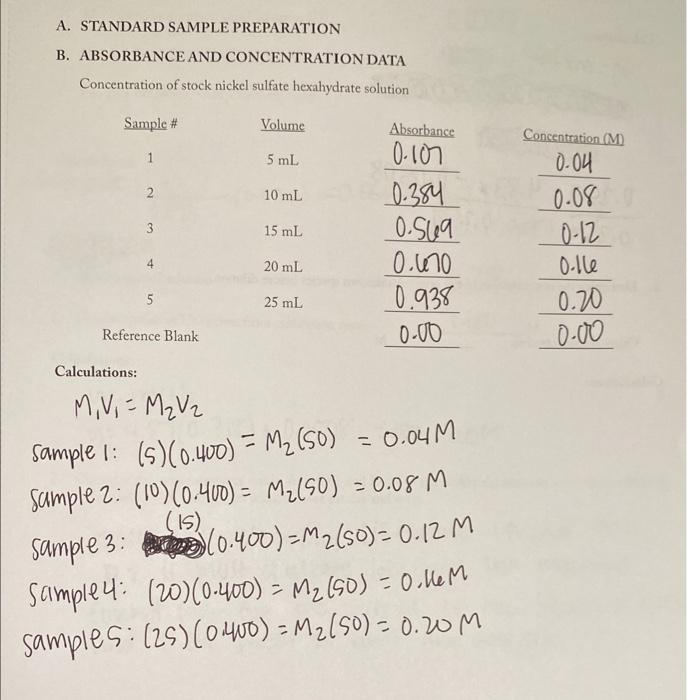

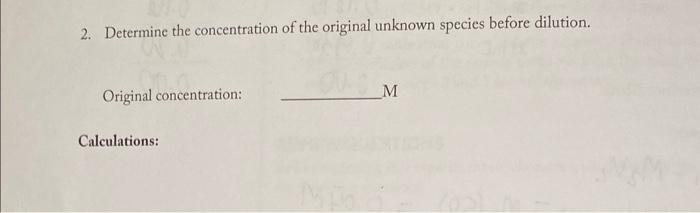
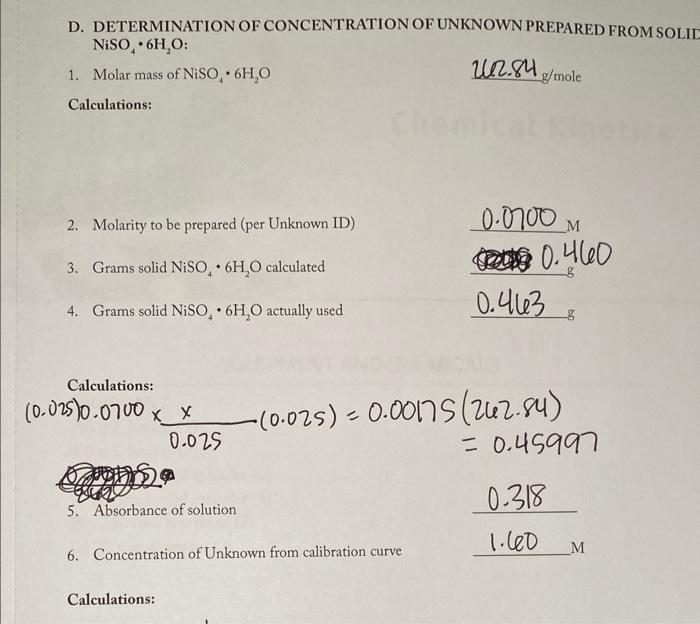
A. STANDARD SAMPLE PREPARATION B. ABSORBANCE AND CONCENTRATION DATA Concentration of stock nickel sulfate hexahydrate solution Calculations: M1V1=M2V2 sample 1: (5)(0.400)=M2(50)=0.04M sample 2: (10)(0.400)=M2(50)=0.08M sample 3: (0.400)(15)=m2(50)=0.12M sample 4: (20)(0.400)=M2(50)=0.16M samples: (25)(0.400)=M2(50)=0.20M C. DETERMINATION OF CONCENTRATION OF LIQUID UNKNOWN: Unknown ID. 240 Absorbance 1. Determine the concentration of the diluted unknown species using the calibration curve. Diluted concentration: M 2. Determine the concentration of the original unknown species before dilution. Original concentration: M Calculations: D. DETERMINATION OF CONCENTRATION OF UNKNOWN PREPARED FROM SOLII NiSO46H2O 1. Molar mass of NiSO46H2O212.84g/mole Calculations: 2. Molarity to be prepared (per Unknown ID) 3. Grams solid NiSO46H2O calculated 4. Grams solid NiSO46H2O actually used 0.463= (0.025)0.07000.025x(0.025)=0.00175=262.84) A. STANDARD SAMPLE PREPARATION B. ABSORBANCE AND CONCENTRATION DATA Concentration of stock nickel sulfate hexahydrate solution Calculations: M1V1=M2V2 sample 1: (5)(0.400)=M2(50)=0.04M sample 2: (10)(0.400)=M2(50)=0.08M sample 3: (0.400)(15)=m2(50)=0.12M sample 4: (20)(0.400)=M2(50)=0.16M samples: (25)(0.400)=M2(50)=0.20M C. DETERMINATION OF CONCENTRATION OF LIQUID UNKNOWN: Unknown ID. 240 Absorbance 1. Determine the concentration of the diluted unknown species using the calibration curve. Diluted concentration: M 2. Determine the concentration of the original unknown species before dilution. Original concentration: M Calculations: D. DETERMINATION OF CONCENTRATION OF UNKNOWN PREPARED FROM SOLII NiSO46H2O 1. Molar mass of NiSO46H2O212.84g/mole Calculations: 2. Molarity to be prepared (per Unknown ID) 3. Grams solid NiSO46H2O calculated 4. Grams solid NiSO46H2O actually used 0.463= (0.025)0.07000.025x(0.025)=0.00175=262.84)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


