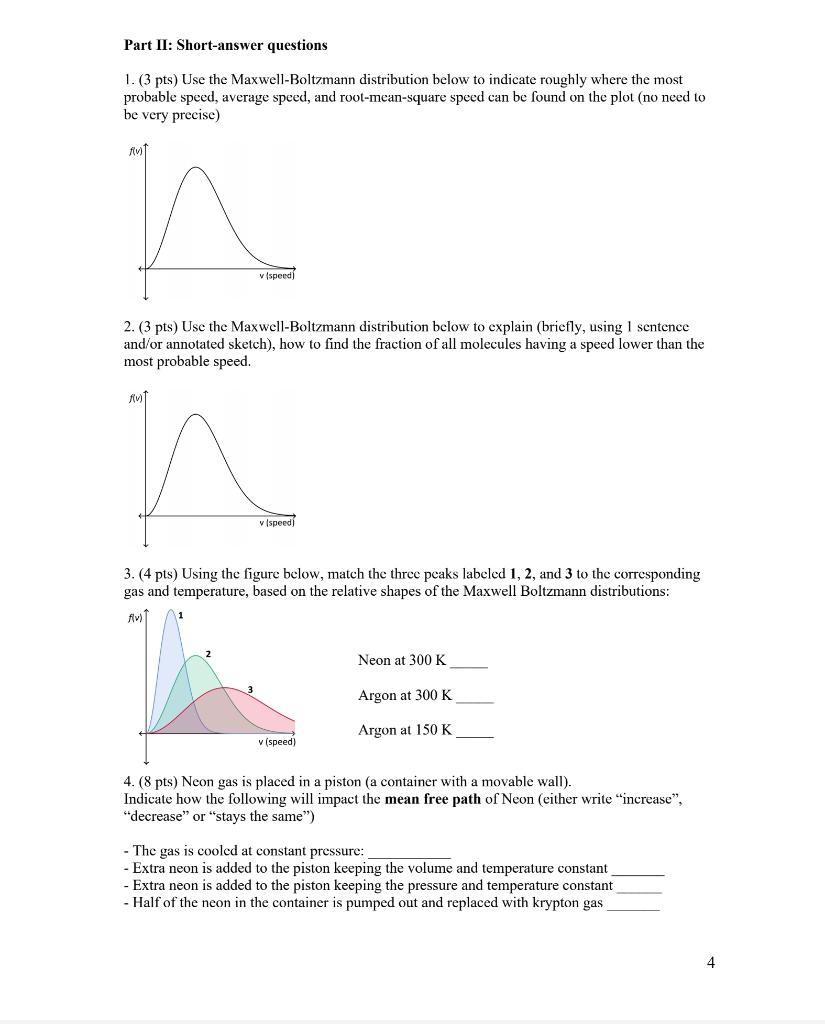
Question: Please help and explain as detail as possible. Thanks Part II: Short-answer questions 1. (3 pts) Use the Maxwell-Boltzmann distribution below to indicate roughly where
Please help and explain as detail as possible. Thanks
Part II: Short-answer questions 1. (3 pts) Use the Maxwell-Boltzmann distribution below to indicate roughly where the most probable speed, average speed, and root-mean-square speed can be found on the plot (no need to be very precise) 2. (3 pts) Use the Maxwell-Boltzmann distribution below to explain (briefly, using 1 sentence and/or annotated sketch), how to find the fraction of all molecules having a speed lower than the most probable speed. 3. (4 pts) Using the figure below, match the three peaks labeled 1, 2, and 3 to the corresponding gas and temperature, based on the relative shapes of the Maxwell Boltzmann distributions: Neon at 300K Argon at 300K Argon at 150K 4. (8pts) Neon gas is placed in a piston (a container with a movable wall). Indicate how the following will impact the mean free path of Neon (either write "increase", "decrease" or "stays the same") - The gas is cooled at constant pressure: - Extra neon is added to the piston keeping the volume and temperature constant - Extra neon is added to the piston keeping the pressure and temperature constant - Half of the neon in the container is pumped out and replaced with krypton gas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


