Question: please help answer 7 and 8 3.Fill the data in the following table: Whad A Concentration of Amino Acids: 4. Prepare a graph of your
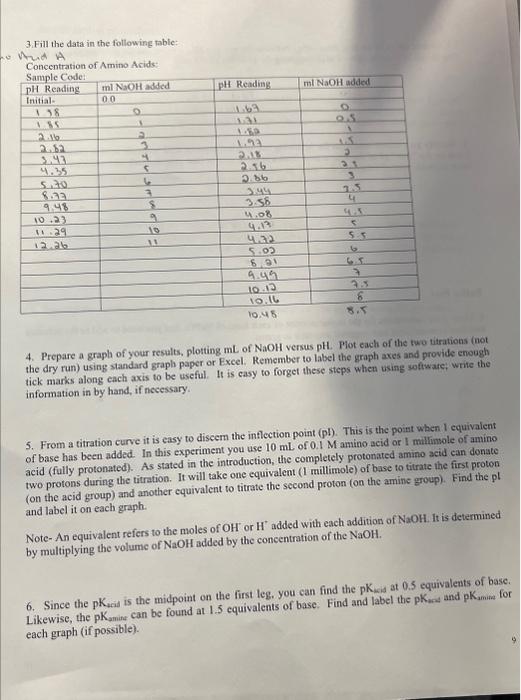

3.Fill the data in the following table: Whad A Concentration of Amino Acids: 4. Prepare a graph of your results, plotting mL of NaOH versus pH. Plot each of the two uitrations (not the dry run) using standard graph paper or Excel. Remember to label the graph axes and provide enough tick marks along each axis to be useful. It is casy to forget these stcps when using woftwate; wrie the information in by hand, if necessary. 5. From a titration curve it is easy to discem the inflection point ( pl). This is the point when 1 equivalent of base has been added. In this experiment you use 10mL of 0.1M amino acid or 1 millmole of amino acid (fully protonated). As stated in the introduction, the completely protonated amino acid can donate two protons during the titration. It will take one equivalent ( 1 millimole) of base to titrate the first proton (on the acid group) and another equivalent to titrate the second proton (on the amine group). Find the pl and label it on each graph. Note- An equivalent refers to the moles of OH+or H+added with each addition of NaOH. is is determined by multiplying the volume of NaOH added by the concentration of the NaOH. 6. Since the pKa is the midpoint on the first leg. you can find the pK wid at 0.5 equivalents of base. Likewise, the pKKamiat can be found at 1.5 equivalents of base. Find and label the pKara and pKamia for each graph (if possible). 7. Calculate the pl by finding the average between the average pK acid value and the average pKaminevalue.. This should be a better method of determining the pl of the amino acid. 8. Identify your amino acid from the following list: glycine, aspartic acid, glutamic acid or phenylalanine. Look up the "true" pl of the amino acid from a reliable source (list your source), and calculate a percent error between your pl value and the "true" pl value. Buffer Part 9. Show your calculation for the molar amount of each form of NaPO4 that you need to add to the solution and then convert them into required amounst in grams. 10. Write down the final pH of your buffer measured by pH-meter. Does the pH determined by Henderson-Hasslebach equation method agree with the desired pH of 7.5 ? If not, can you explain why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


