Question: Please help answer all subparts, im not sure if the ones I have already are correct. Thank you. 5-7. What is the major drawback of

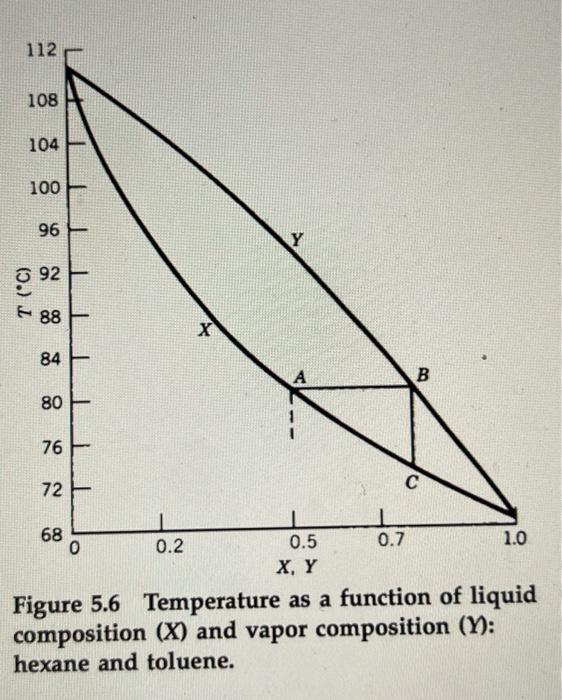
5-7. What is the major drawback of trying to distill a 500 I below 200BC ? 5-8. How might you separate the mixture discussed in question 5-6 if distillation were unsuccessful? Explain your choice. 5-9. If starting with an equal mixture of hexane and toluene, approximate the composition of hexane if the vapor at 94IC is condensed to a liquid using the data presented in Figure 5.6. The approximate composition of hexane if the vapor at 94 degrees is condensed to a liquid would be 0.2. 5-10. Why do simple distillations require that the components of the mixture to be separated have boiling points that are separated by 40EC or more? If there is a larger difference in boiling point (above 40 degrees) simple distillation is used, this is because if there were a smaller difference in boiling point the mixture of components would not separate into two pure substances. Therefore, there is a possibility that the components may mix up. 5-11. Which constituent of an equimolar mixture makes the larger contribution to the vapor pressure of the mixture, the higher or lower boiling component? Explain. Between hexane and toluene, hexane makes a larger contribution to vapor pressure. The lower boiling component contributes to a larger contribution due to the substance being more volatile.| Figure 5.6 Temperature as a function of I1quia composition (X) and vapor composition (Y): hexane and toluene
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


