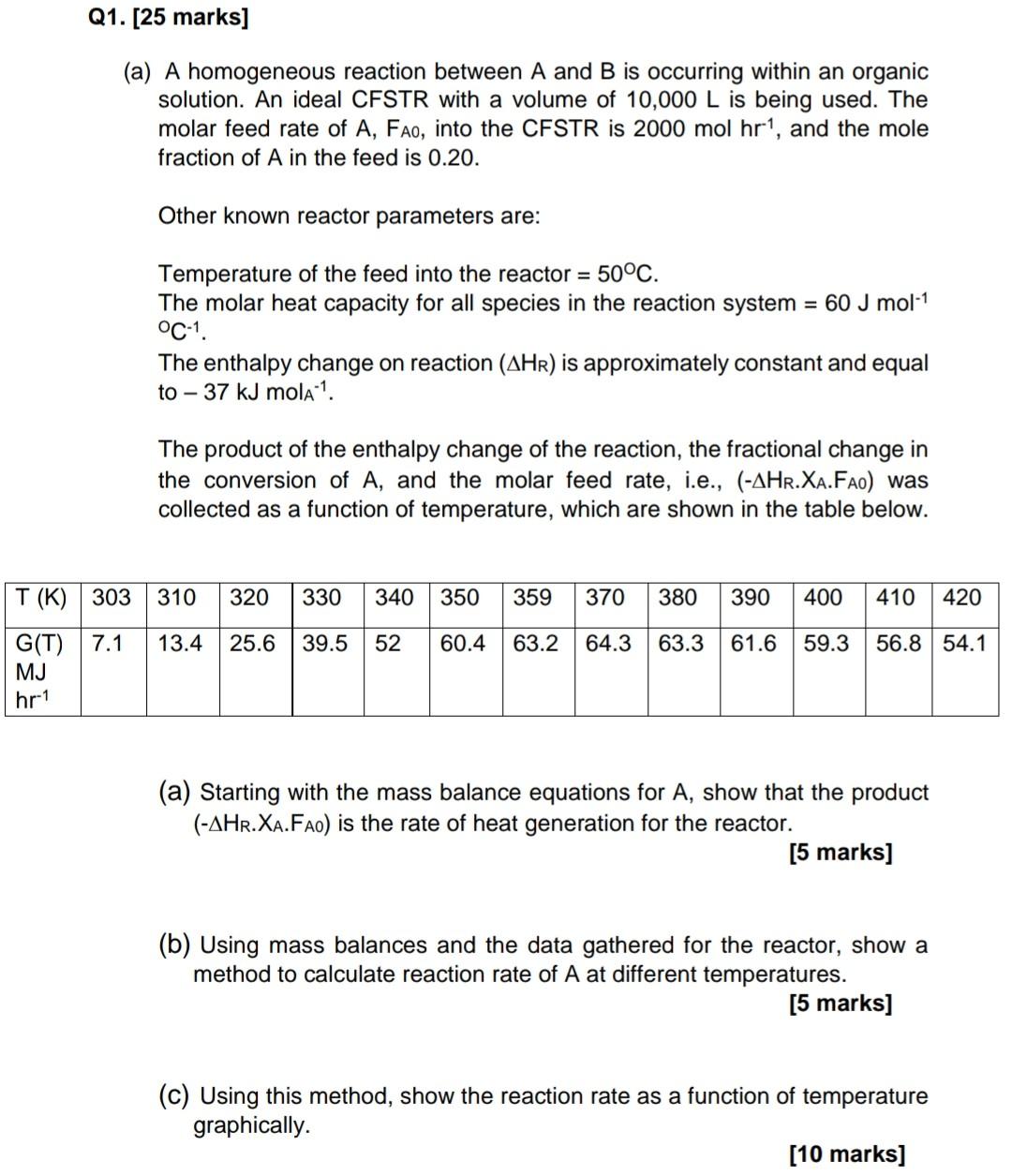
Question: PLEASE HELP ANSWER B AND C ONLY. I WILL DISLIKE IF SPAM Cancel no need for it anymore thanks Q1. [25 marks] (a) A homogeneous
PLEASE HELP ANSWER B AND C ONLY. I WILL DISLIKE IF SPAM
Cancel no need for it anymore thanks
Q1. [25 marks] (a) A homogeneous reaction between A and B is occurring within an organic solution. An ideal CFSTR with a volume of 10,000 L is being used. The molar feed rate of A, Fao, into the CFSTR is 2000 mol hr!, and the mole fraction of A in the feed is 0.20. Other known reactor parameters are: = Temperature of the feed into the reactor = 50C. The molar heat capacity for all species in the reaction system = 60 J mol-1 C-1 The enthalpy change on reaction (AHR) is approximately constant and equal to - 37 kJ mola ?. The product of the enthalpy change of the reaction, the fractional change in the conversion of A, and the molar feed rate, i.e., (-AHR.XA.Fao) was collected as a function of temperature, which are shown in the table below. 310 320 330 340 350 359 370 380 390 400 410 420 T (K) 303 G(T) 7.1 MJ hr-1 13.4 25.6 39.5 52 60.4 63.2 64.3 63.3 61.6 59.3 56.8 54.1 (a) Starting with the mass balance equations for A, show that the product (-AHR.XA.Fao) is the rate of heat generation for the reactor. [5 marks] (b) Using mass balances and the data gathered for the reactor, show a method to calculate reaction rate of A at different temperatures. [5 marks] (C) Using this method, show the reaction rate as a function of temperature graphically. [10 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


