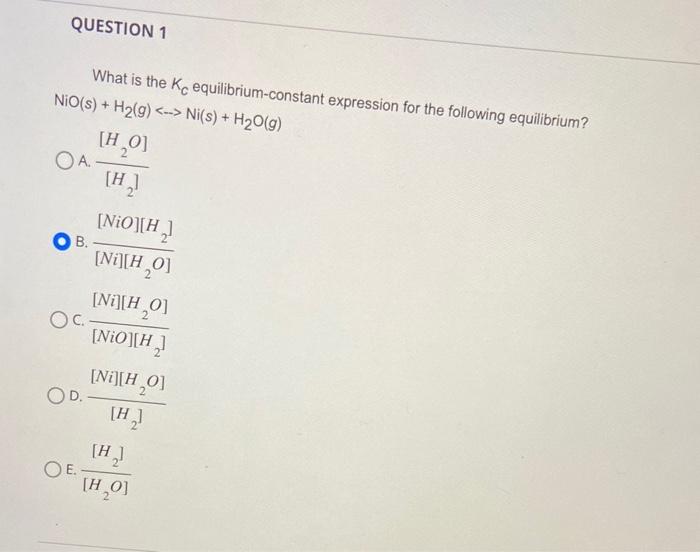
Question: please help answer, im stuck What is the Kc equilibrium-constant expression for the following equilibrium? NiO(s)+H2(g)Ni(s)+H2O(g) A. [H2][H2O] B. [Ni][H2O][NiO][H2] C. [NiO][H2][Ni][H2O] D. [H2][Ni][H2O] E.
![for the following equilibrium? NiO(s)+H2(g)Ni(s)+H2O(g) A. [H2][H2O] B. [Ni][H2O][NiO][H2] C. [NiO][H2][Ni][H2O] D.](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8e4e0ebffa_55266f8e4e0a16c2.jpg)
![[H2][Ni][H2O] E. [H2O][H2] For a general chemical reaction, if you have a](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8e4e182dcf_55366f8e4e12cbc6.jpg)
What is the Kc equilibrium-constant expression for the following equilibrium? NiO(s)+H2(g)Ni(s)+H2O(g) A. [H2][H2O] B. [Ni][H2O][NiO][H2] C. [NiO][H2][Ni][H2O] D. [H2][Ni][H2O] E. [H2O][H2] For a general chemical reaction, if you have a reaction quotient equal to 3.5102 and an equilibrium constant equal to 2.1102, the reaction will A. shift towards the reactants B. shift towards the products C. stay the same For the following reaction CO(g)+H2O(g)CO2(g)+H2(g) Suppose 0.020mol of CO and 0.020mol of H2O are added to a 1.0- L container. If molofCO2 are present at equilibrium, how many moles of CO are present at equilibrium? A. 2x B. 0.0202x C. 0.020+x D. 0.020x E. x
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


