Question: please help answer part A-C Consider tho reaction: K=4.0 A(g)2B(g) Express your answers in bar to two significant figures separated by a comma Find the
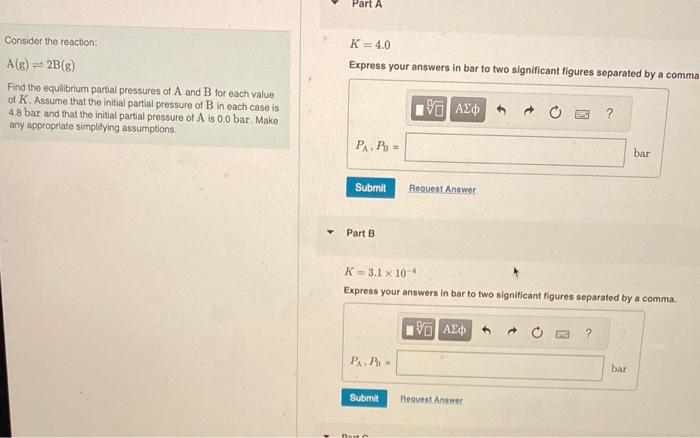
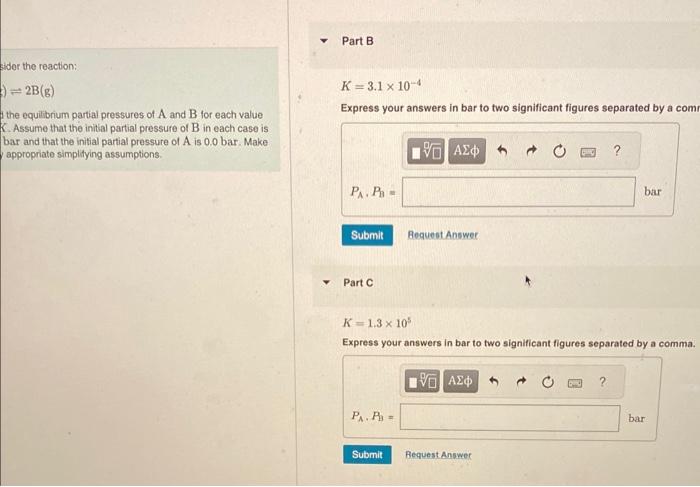
Consider tho reaction: K=4.0 A(g)2B(g) Express your answers in bar to two significant figures separated by a comma Find the equilibrium partial pressures of A and B tor each value of K. Assume that the initial partial pressure of B in each case is 4.8 bar and that the initial partial pressure of A is 0.0 bar. Make any appropriate simplitying assumptions. Part B K=3.1104 Express your answers in bar to two signilicant figures separated bv a comma. bider the reaction: )=2B(g) K=3.1104 the equilibrium partial pressures of A and B for each value Express your answers in bar to two significant figures separated by a com. K. Assume that the initial partial pressure of B in each case is bar and that the initial partial pressure of A is 0.0 bar. Make appropriate simplitying assumptions. PartCK=1.3105 Express your answers in bar to two significant figures separated by a comma
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


