Question: 1. This problem concerns the isothermal Joule-Thomson coefficient, ,, which is different from the usual Joule-Thomson coefficient, . The definitions are: AH-0! = (37)
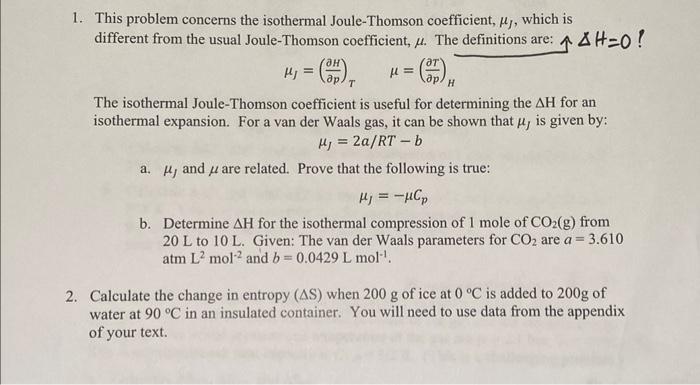

1. This problem concerns the isothermal Joule-Thomson coefficient, ,, which is different from the usual Joule-Thomson coefficient, . The definitions are: AH-0! = (37) = (37) T H The isothermal Joule-Thomson coefficient is useful for determining the AH for an isothermal expansion. For a van der Waals gas, it can be shown that , is given by: Hj = 2a/RT-b a. , and are related. Prove that the following is true: H = -HCp b. Determine AH for the isothermal compression of 1 mole of CO(g) from 20 L to 10 L. Given: The van der Waals parameters for CO are a = atm L mol2 and b=0.0429 L mol-. = 3.610 2. Calculate the change in entropy (AS) when 200 g of ice at 0 C is added to 200g of water at 90 C in an insulated container. You will need to use data from the appendix of your text. 3. This question is about calculating the absolute entropy from experimental data. We saw in class that this can be done by combining AS for heating with AS for phase changes. In this problem, the integral you need for AS of heating is evaluated graphically as the area under a curve. You can approximate this area as a sum of rectangles. I recommend using Excel to do so. Calculate the standard molar entropy (Sm) of carbon disulfide (CS) at 298 K from the heat capacity data below and the enthalpy of fusion at Tr= 161.11 K: AfusHo =4389 J/mol. Below 15 K, assume that the molar heat capacity obeys the relationship CpmaT, where a is a constant. You can evaluate a from one of the data points. T(K) 15.05 20.15 29.76 42.22 57.52 75.54 89.37 Cpm (J K- mol-1) 6.90 12.01 20.75 29.16 35.56 40.04 43.14 Cpm (JK- mol-) 45.94 48.49 50.50 52.63 56.62 161.11-298 75.48 T(K) 99.00 108.93 119.91 131.54 156.83 4. The combustion of n-propane occurs according to the chemical equation: C3Hs(g) +502(g) 3CO2(g) + 4HO(1) a. Calculate A,H, A.S, and A.G for the combustion of 1 mole of n-propane at T = 298 K. b. Calculate A,H, A,S and A,G for the combustion of 1 mole of n-propane at T = 348 K. You may assume that Cpm for each reactant and product is constant over the temperature range given. The data needed can be found in the appendix of your text. no heat transfer! q=0 5. One mole of an ideal gas is expanded adiabatically against a constant external pressure of pex = 1.4 bar until the internal pressure of the gas and the external pressure are equal. The initial pressure and temperature of the gas are pi= 2.1 bar, Ti= 400 K. The constant-pressure heat capacity is Cv.m 3/2 R. (This is a challenging problem.) E a. Calculate ASsys, ASsur, and AStot. b. State whether your answer is reasonable in view of the 2nd Law of Thermodynamics. Provide your reasoning. PivineTi PFVF=nRTF
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


