Question: Please help answering these questions. Not sure why I keep on getting them wrong. Thank you 1. For the given anion, draw all significant resonance
 Please help answering these questions. Not sure why I keep on getting them wrong. Thank you
Please help answering these questions. Not sure why I keep on getting them wrong. Thank you
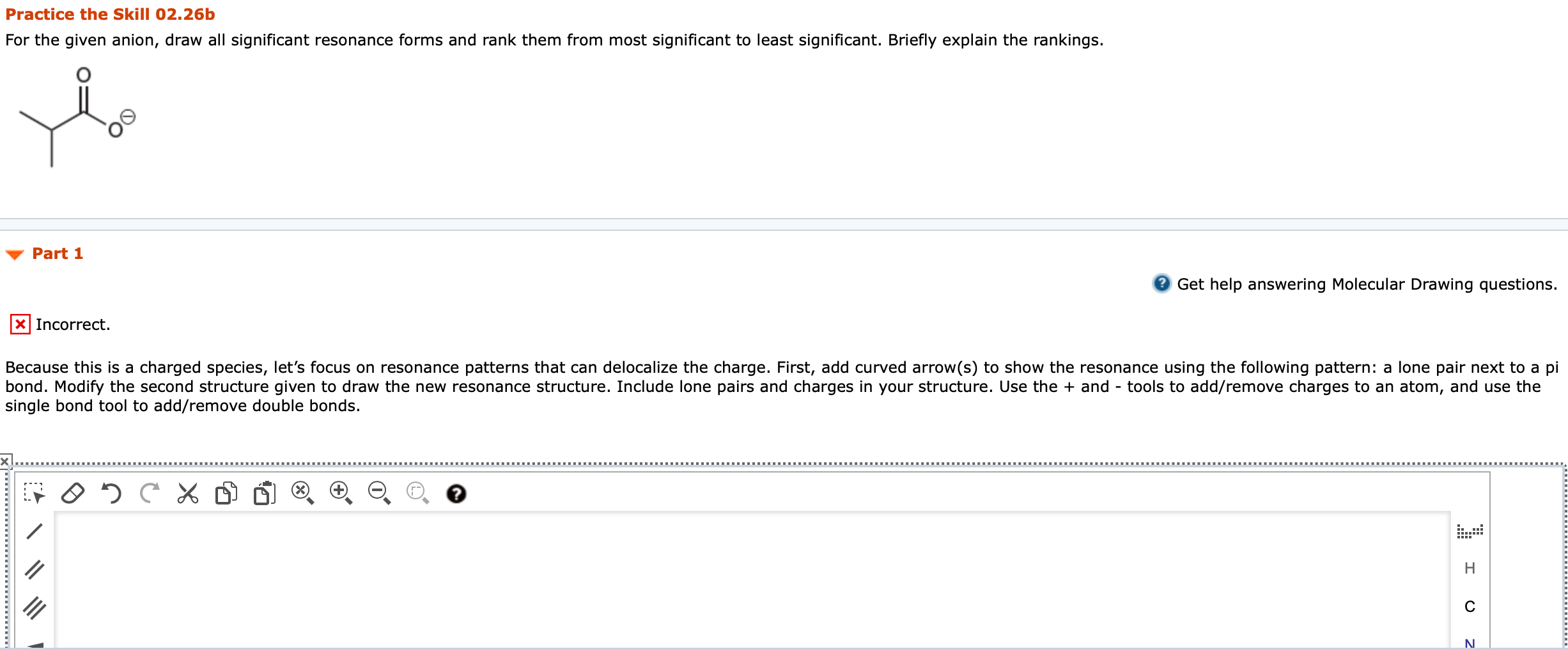
1. For the given anion, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. Because this is a charged species, lets focus on resonance patterns that can delocalize the charge. First, add curved arrow(s) to show the resonance using the following pattern: a lone pair next to a pi bond. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.
e given anion, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings.
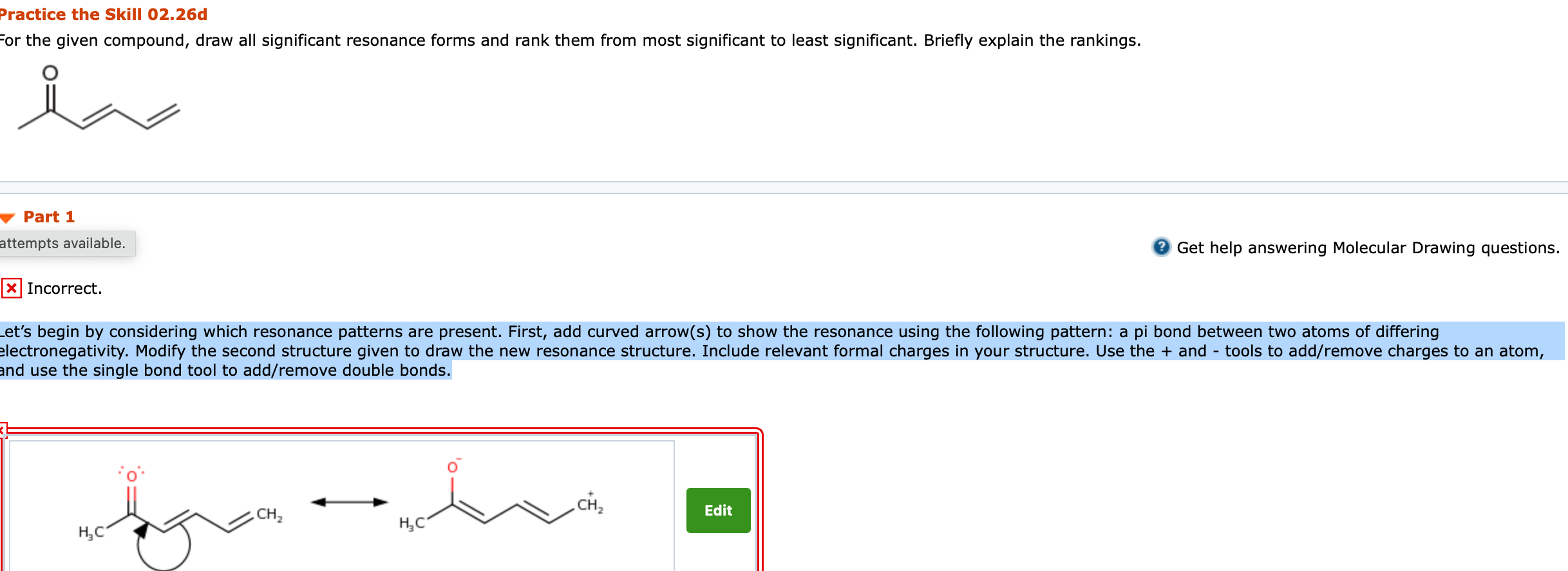
2. For the given compound, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. Lets begin by considering which resonance patterns are present. First, add curved arrow(s) to show the resonance using the following pattern: a pi bond between two atoms of differing electronegativity. Modify the second structure given to draw the new resonance structure. Include relevant formal charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.
Practice the Skill 02.26b For the given anion, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. Part 1 2 Get help answering Molecular Drawing questions. Incorrect. Because this is a charged species, let's focus on resonance patterns that can delocalize the charge. First, add curved arrow(s) to show the resonance using the following pattern: a lone pair next to a pi bond. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. 95 . II H III N Practice the Skill 02.26d For the given compound, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. Part 1 attempts available. Get help answering Molecular Drawing questions. X Incorrect. Let's begin by considering which resonance patterns are present. First, add curved arrow(s) to show the resonance using the following pattern: a pi bond between two atoms of differing electronegativity. Modify the second structure given to draw the new resonance structure. Include relevant formal charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. CH, CH Edit HC " HC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


