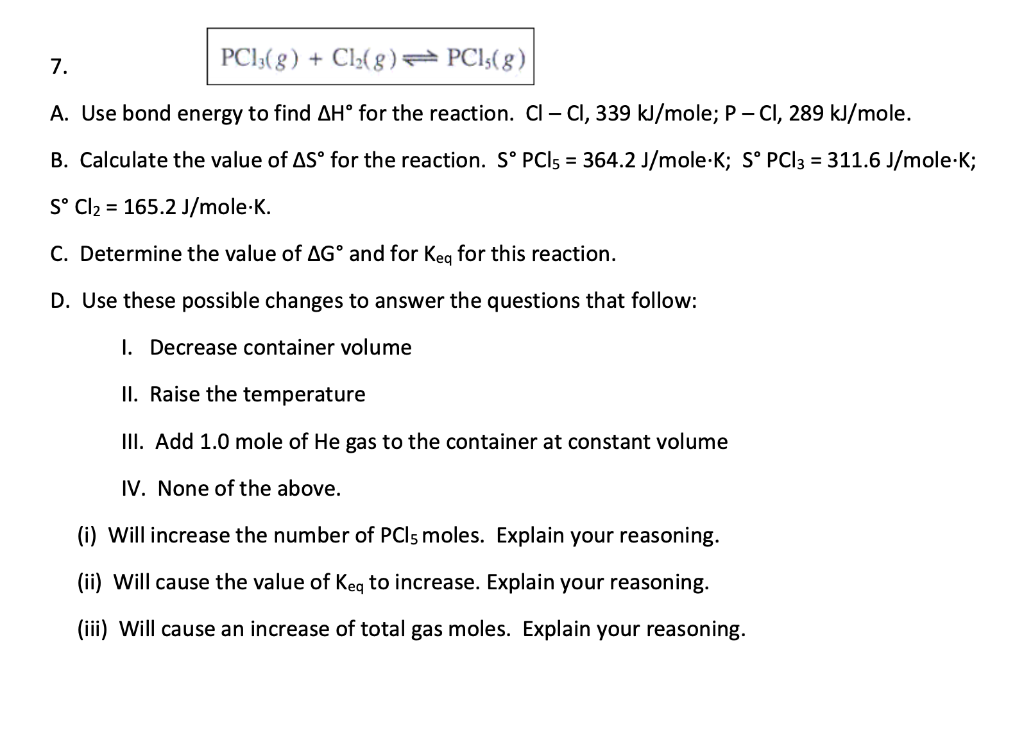
Question: Please help ASAP 7. PCI;(8) + Cl2(8) 7 PCl:(8) A. Use bond energy to find AH for the reaction. Cl CI, 339 kJ/mole; P-CI, 289
Please help ASAP
7. PCI;(8) + Cl2(8) 7 PCl:(8) A. Use bond energy to find AH for the reaction. Cl CI, 339 kJ/mole; P-CI, 289 kJ/mole. B. Calculate the value of AS for the reaction. S PCI5 = 364.2 J/mole.K; S PCl3 = 311.6 J/mole-K; S Cl2 = 165.2 J/mole.K. C. Determine the value of AG and for Keq for this reaction. D. Use these possible changes to answer the questions that follow: 1. Decrease container volume II. Raise the temperature III. Add 1.0 mole of He gas to the container at constant volume IV. None of the above. (i) Will increase the number of PCls moles. Explain your reasoning. (ii) Will cause the value of Key to increase. Explain your reasoning. (iii) Will cause an increase of total gas moles. Explain your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


