Question: PLEASE HELP ASAP WILL RATE HIGH For this assignment, we need to make 2 liters of 10X PBS solution. PBS stands for phosphate buffered saline,
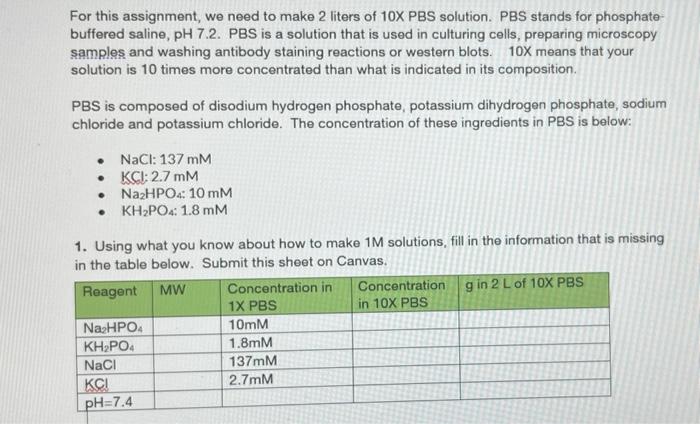
For this assignment, we need to make 2 liters of 10X PBS solution. PBS stands for phosphate buffered saline, pH 7.2. PBS is a solution that is used in culturing cells, preparing microscopy samples and washing antibody staining reactions or western blots. 10X means that your solution is 10 times more concentrated than what is indicated in its composition. PBS is composed of disodium hydrogen phosphate, potassium dihydrogen phosphate, sodium chloride and potassium chloride. The concentration of these ingredients in PBS is below: NaCl: 137 mM KCI: 2.7 mm NazHPO4: 10 mm KH2PO4: 1.8 mm 1. Using what you know about how to make 1M solutions, fill in the information that is missing in the table below. Submit this sheet on Canvas. Reagent MW Concentration in Concentration g in 2 L of 10X PBS 1X PBS in 10X PBS Na2HPO4 10mm KH2PO4 1.8mm NaCl 137mm 2.7mM pH=7.4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


