Question: please help asap will thumbs up 2. A certain vinegar solution contains 52,4g/L of ethanoic acid. If Ka=1.79x 105 for ethanoic acid, calculate the pH
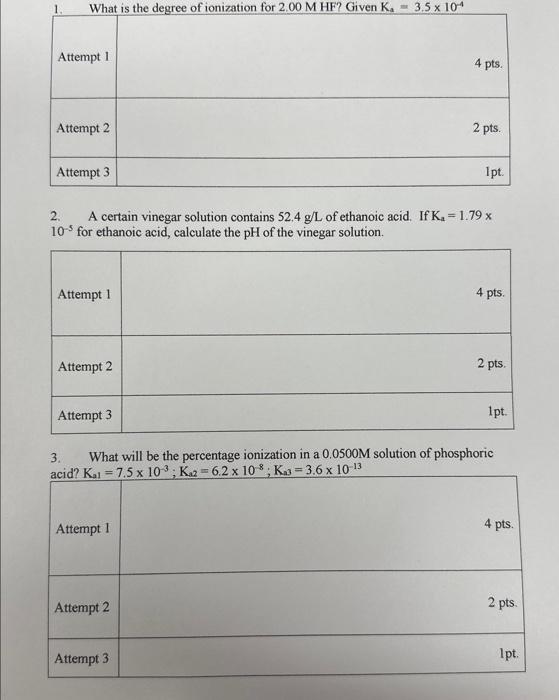
2. A certain vinegar solution contains 52,4g/L of ethanoic acid. If Ka=1.79x 105 for ethanoic acid, calculate the pH of the vinegar solution. 3. What will be the percentage ionization in a 0.0500M solution of phosphoric
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


