Question: please help. calculate the value of Kp The decomposition of nitrous oxide in glass or silica vessels is generally a homogeneous second-order reaction. In the
please help. calculate the value of Kp
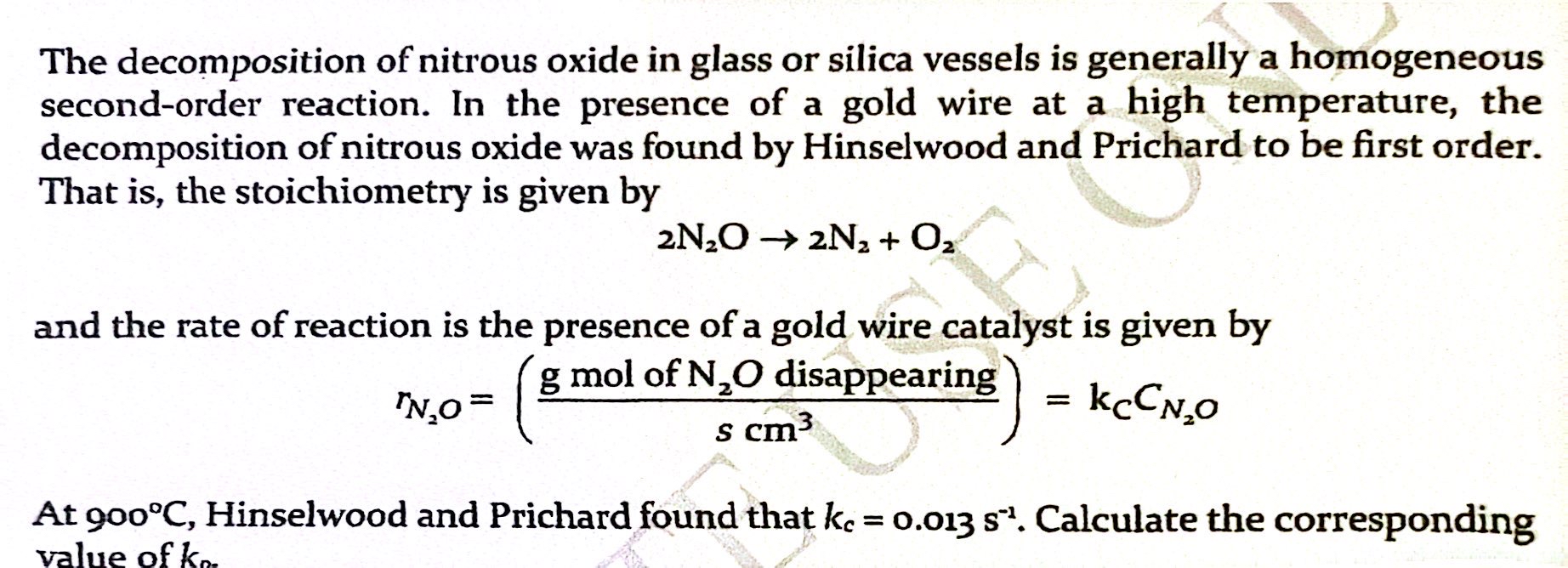
The decomposition of nitrous oxide in glass or silica vessels is generally a homogeneous second-order reaction. In the presence of a gold wire at a high temperature, the decomposition of nitrous oxide was found by Hinselwood and Prichard to be first order. That is, the stoichiomet1Y is given by 2N20 2Nz + 02 and the rate of reaction is the presence ofa gold wire catalyst is given by g mol of NZO disappearing rN20 = 3 s cm = kccN0 At 9000C, Hinselwood and Prichard found that kc = 0.013 Sl. Calculate the corresponding
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


