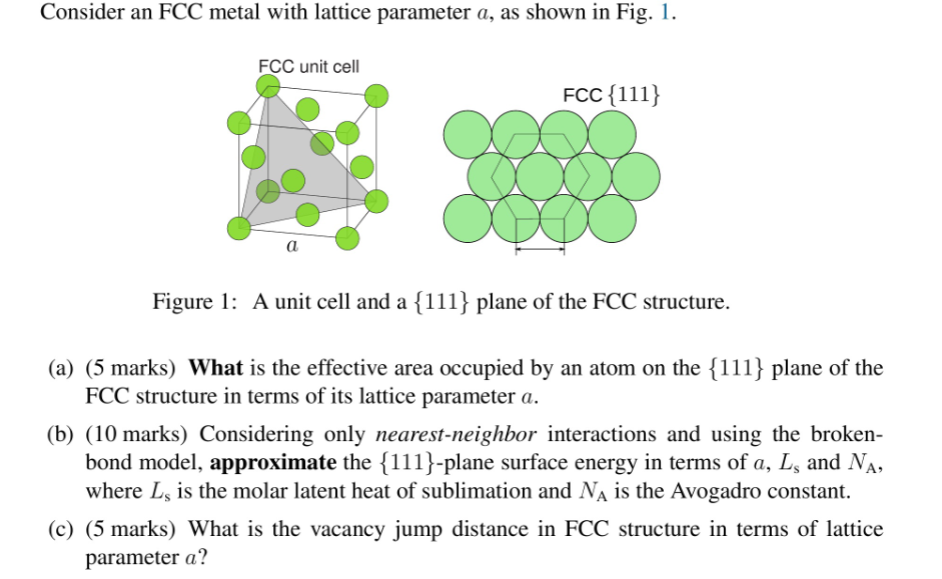
Question: please help! Consider an FCC metal with lattice parameter a, as shown in Fig. 1. FCC unit cell FCC{111} a Figure 1: A unit cell
please help!
Consider an FCC metal with lattice parameter a, as shown in Fig. 1. FCC unit cell FCC{111} a Figure 1: A unit cell and a {111} plane of the FCC structure. (a) (5 marks) What is the effective area occupied by an atom on the {111} plane of the FCC structure in terms of its lattice parameter a. (b) (10 marks) Considering only nearest-neighbor interactions and using the broken- bond model, approximate the {111}-plane surface energy in terms of a, L, and Na, where L, is the molar latent heat of sublimation and N is the Avogadro constant. (c) (5 marks) What is the vacancy jump distance in FCC structure in terms of lattice parameter a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


