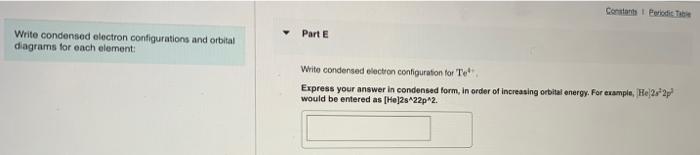
Question: please help Constants Periodic Part E Write condensed electron configurations and orbital diagrams for each element: Write condensed electron configuration for Te Express your answer
![He 2.2 would be entered as [He]2s22p2. Part F Choose the orbital](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9706aec101_27466f9706a946f3.jpg)
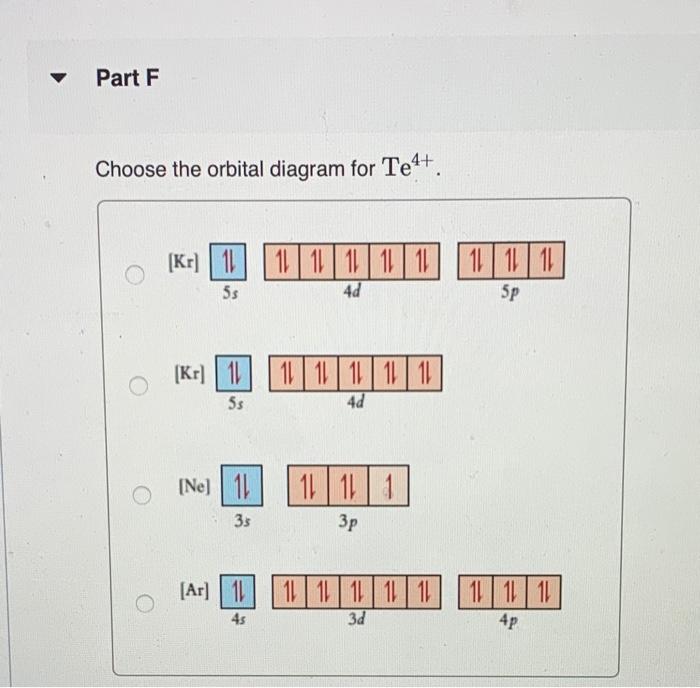
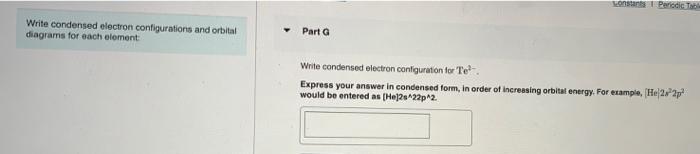
Constants Periodic Part E Write condensed electron configurations and orbital diagrams for each element: Write condensed electron configuration for Te Express your answer in condensed form, in order of increasing orbital energy. For example, He 2.2 would be entered as [He]2s22p2. Part F Choose the orbital diagram for Te4+. [Kr) 11 1111111111111111 5p 5s 4d [Kr) 111111111111 5s 4d [Nel 11 11111 33 o [Ar] 11 45 11 11 11 11 11 11111111111 3d 4p sonus Periodic Tab Write condensed electron configurations and orbital diagrams for each element Part G Write condensed electron configuration for Te. Express your answer in condensed form, in order of increasing orbital energy. For example, He22p would be entered as [Hej2s22p2. Part H Choose the orbital diagram for Tez- [Ne) 11 3s | 11 | 111 3p [Kr] 11111111111111111 5s 5p 4d (Ne) 1 1 1 1 ) 1111 3p 3s [Kr] 111111111111 5s 4d
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


