Question: please help cpuld ypu answer both the questions please A 0.0800kg sample of a gas is heated from 25C to 225C. During this process, 340J
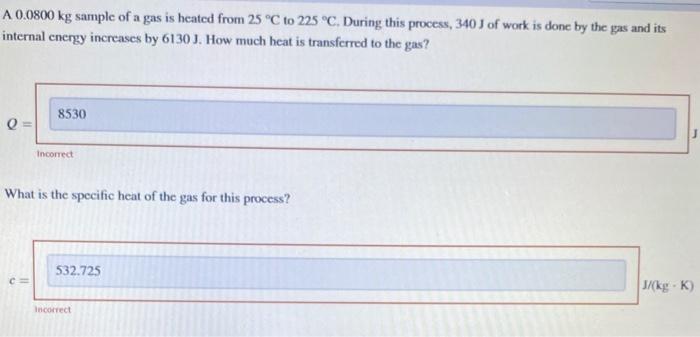
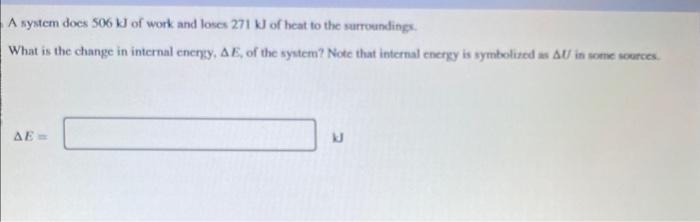
A 0.0800kg sample of a gas is heated from 25C to 225C. During this process, 340J of work is done by the gas and its internal energy increases by 6130 J. How much heat is transferred to the gas? What is the specific heat of the gas for this process? A system does 506kJ of work and loses 271kJ of heat to the surruendings. What is the change in internal eneryy, E, of the system? Note that internal energy is symbolited as U in sonte sources
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


