Question: please help Dinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reaction is first order in dinitrogen pentoxide and
please help 

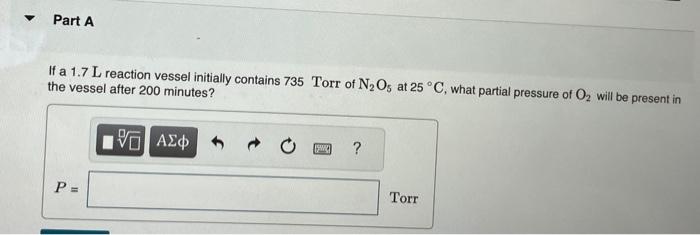
Dinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reaction is first order in dinitrogen pentoxide and has a half-life of 2.81h at 25C. If a 1.7 L reaction vessel initially contains 735 Torr of N2O5 at 25C, what partial pressure of O2 will be present in the vessel after 200 minutes
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


