Question: Please help, do these answers look correct? Hydrogen iodide decomposes via a second-order process to produce hydrogen and iodine according to the following chemical equation
Please help, do these answers look correct?
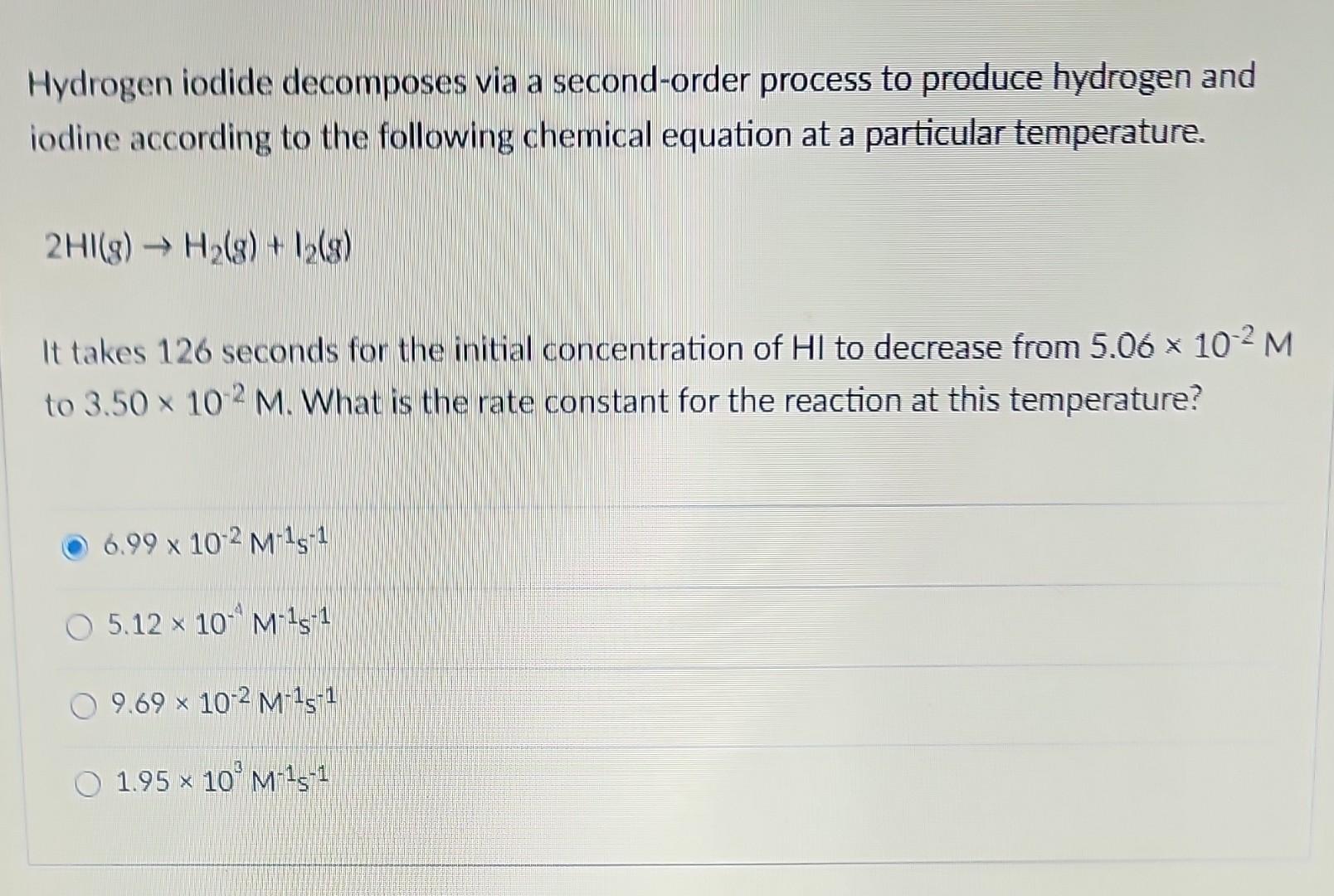

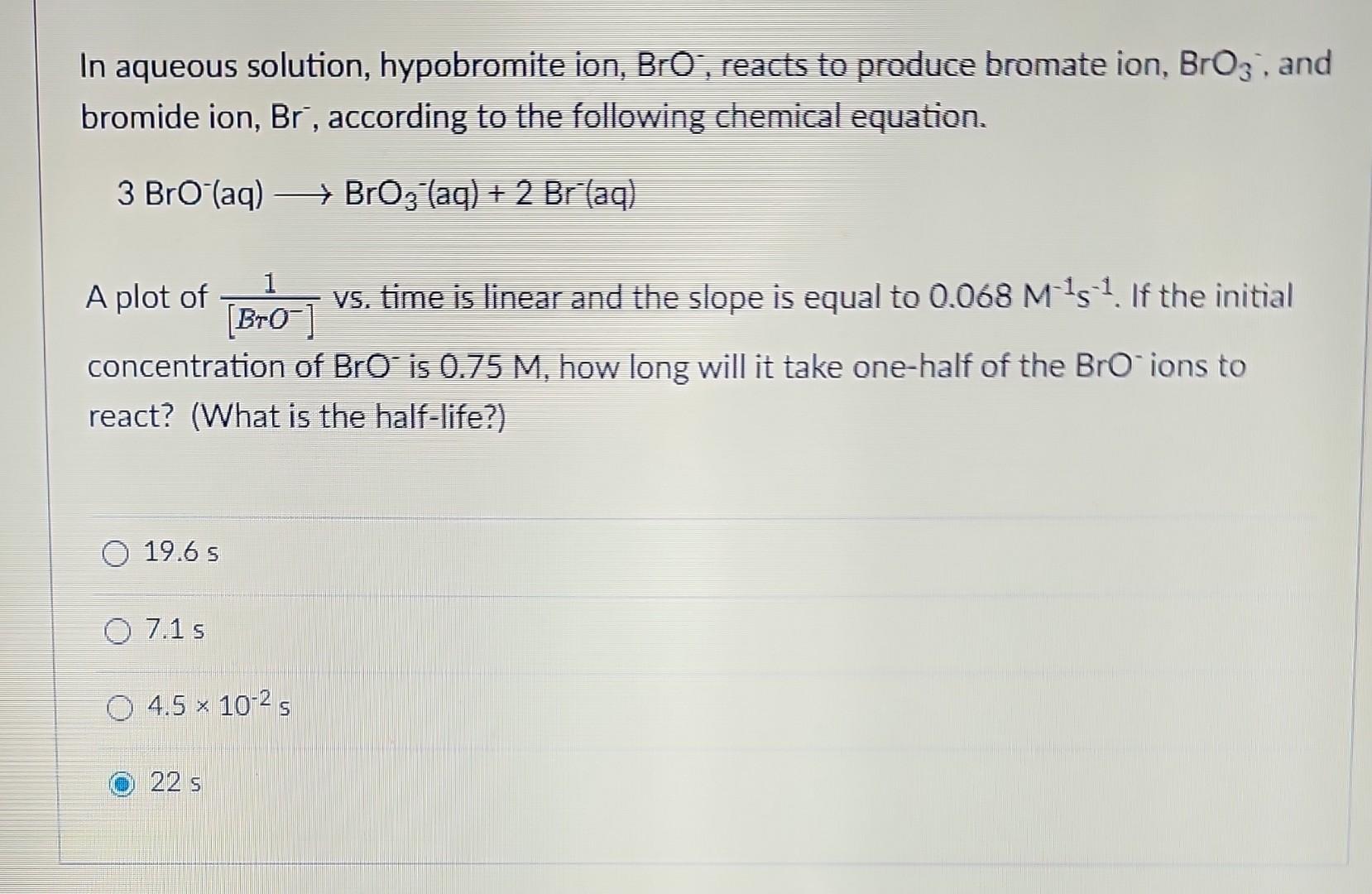
Hydrogen iodide decomposes via a second-order process to produce hydrogen and iodine according to the following chemical equation at a particular temperature. 2HI(g)H2(g)+I2(g) It takes 126 seconds for the initial concentration of HI to decrease from 5.06102M to 3.50102M. What is the rate constant for the reaction at this temperature? 6.99102M1s15.12104M1s19.69102M1s11.95103M1s1 A particular first-order reaction has a rate constant of 1.14102s1 at 25.0C. What is the magnitude of k at 75.0C if Ea=85.6kJ/mol ? (Hint: use the Arrhenius 2-point equation and make sure you convert the temperatures to K ) 1.92104s1670s11.62104s13.85106s11.36102s1 In aqueous solution, hypobromite ion, BrO; reacts to produce bromate ion, BrO3, and bromide ion, Br, according to the following chemical equation. 3BrO(aq)BrO3(aq)+2Br(aq) A plot of [BrO]1vs. time is linear and the slope is equal to 0.068M1s1. If the initial concentration of BrOis 0.75M, how long will it take one-half of the BrOions to react? (What is the half-life?) 19.65 7.15 4.5102s 225
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


