Question: please help. dont get answer from somewhere else or you dont know how to solve. i need also how to integrate Problem 1 (100 points):
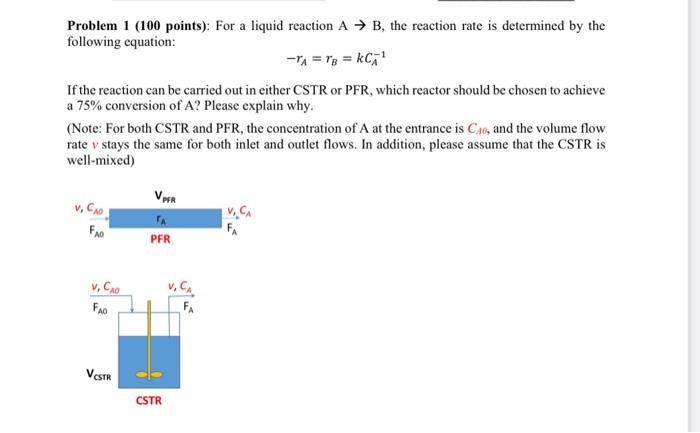
Problem 1 (100 points): For a liquid reaction A B, the reaction rate is determined by the following equation: -r1 = rs = KCR If the reaction can be carried out in either CSTR or PFR, which reactor should be chosen to achieve a 75% conversion of A? Please explain why. (Note: For both CSTR and PFR, the concentration of A at the entrance is CA, and the volume flow rate v stays the same for both inlet and outlet flows. In addition, please assume that the CSTR is well-mixed) VPER v, FAO PER V. CAO FAO VESTR CSTR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


