Question: please help Draw a resonance structure that places a pi bond in a different position. Include all lone pairs in your structure. Incorrect, 1 attempt
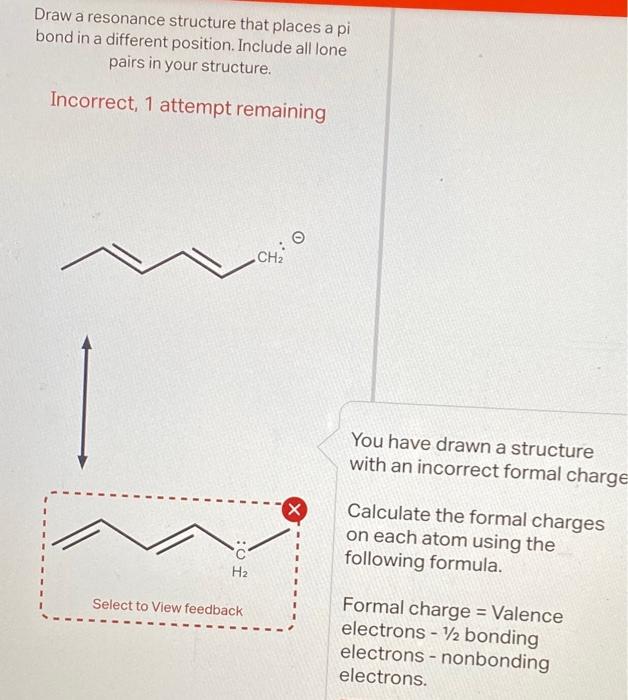
Draw a resonance structure that places a pi bond in a different position. Include all lone pairs in your structure. Incorrect, 1 attempt remaining You have drawn a structure with an incorrect formal charge Calculate the formal charges on each atom using the following formula. Formal charge = Valence electrons 1/2 bonding electrons - nonbonding electrons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


