Question: please help. due at 12 on 2.21. will rate Phosphorus pentachloride decomposes according to the chemical equation PCl5(g)PCl3(g)+Cl2(g)Kc=1.80at250C A 0.3200 mol sample of PCl5(g) is
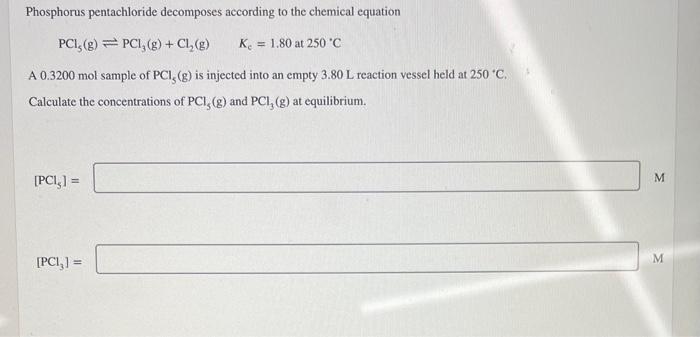
Phosphorus pentachloride decomposes according to the chemical equation PCl5(g)PCl3(g)+Cl2(g)Kc=1.80at250C A 0.3200 mol sample of PCl5(g) is injected into an empty 3.80L reaction vessel held at 250C. Calculate the concentrations of PCl5(g) and PCl3(g) at equilibrium. [PCl5]=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


