Question: Please help/ explain- thank you! Background: What is the initial pressure of H2S(g) in the flask? Express your answer numerically in atmospheres. An empty 5.00-L
Please help/ explain- thank you!
Background:
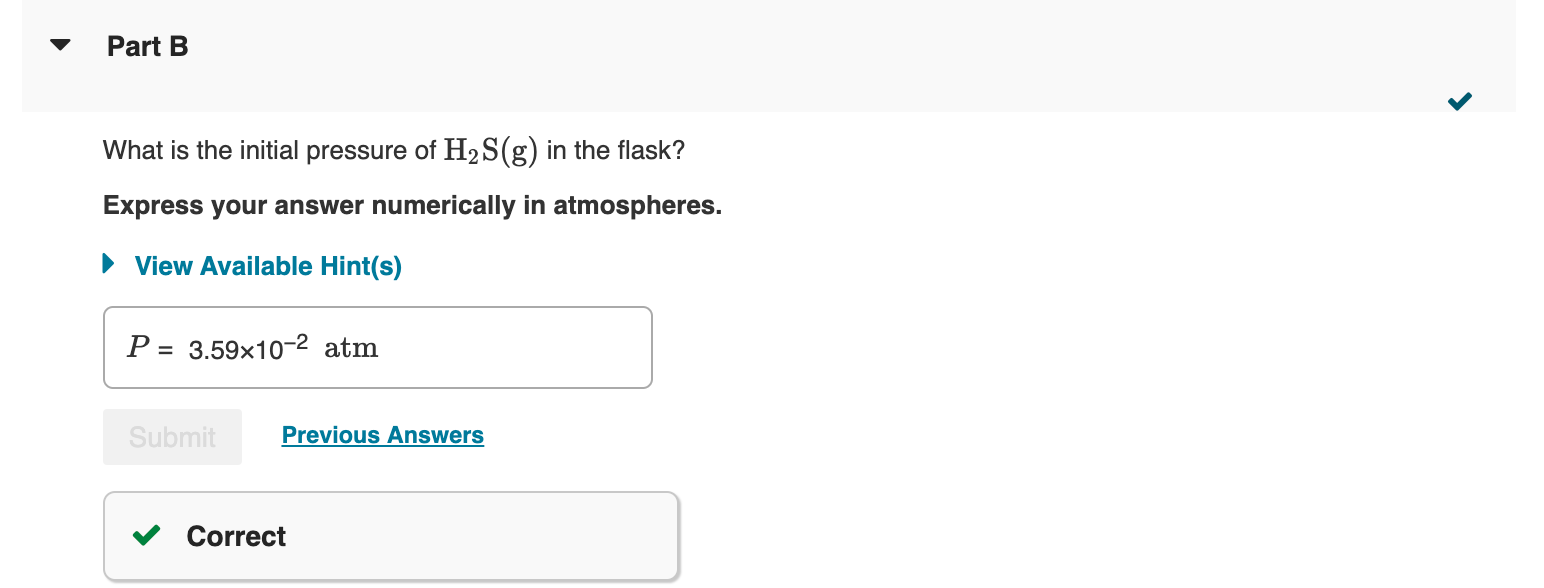

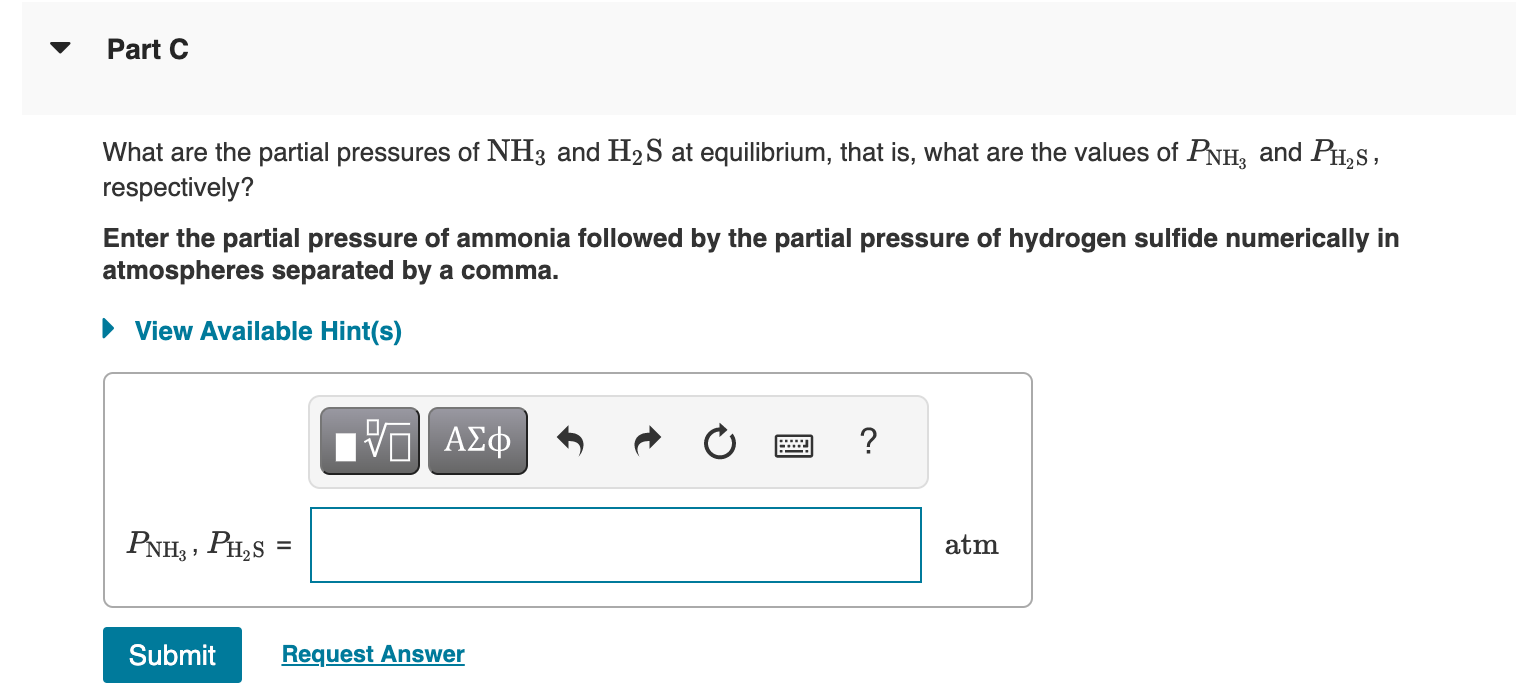
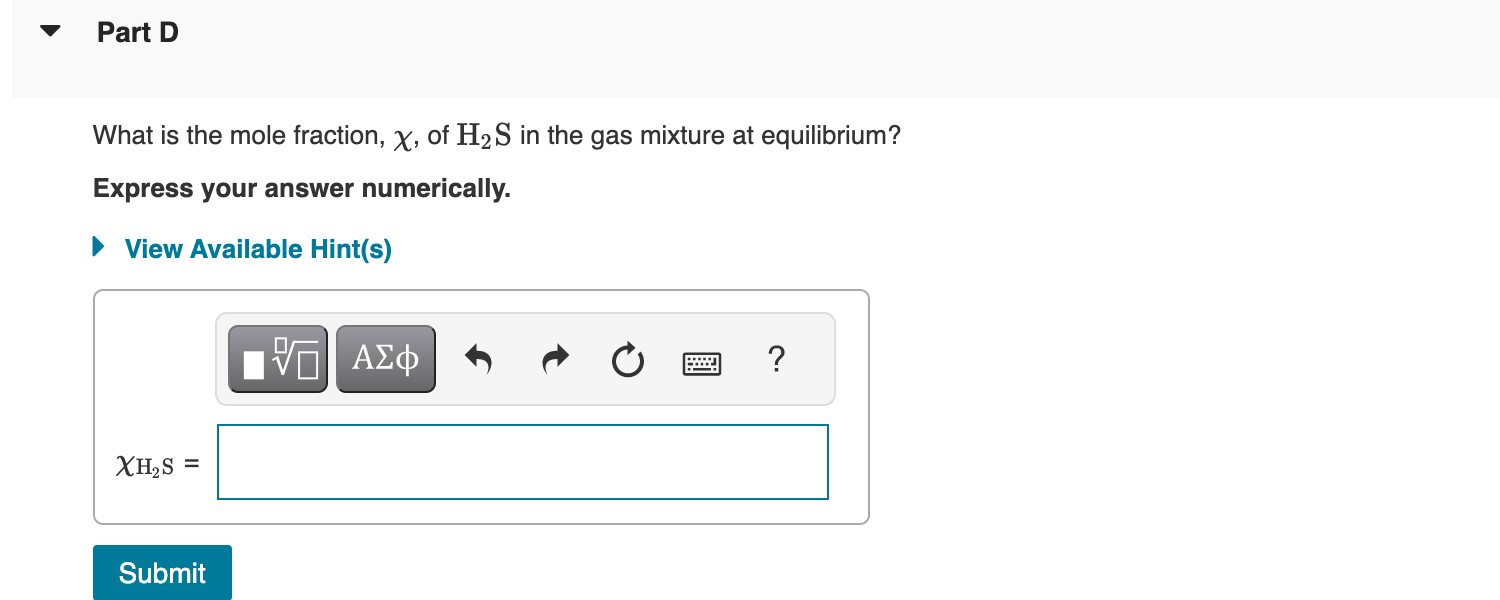
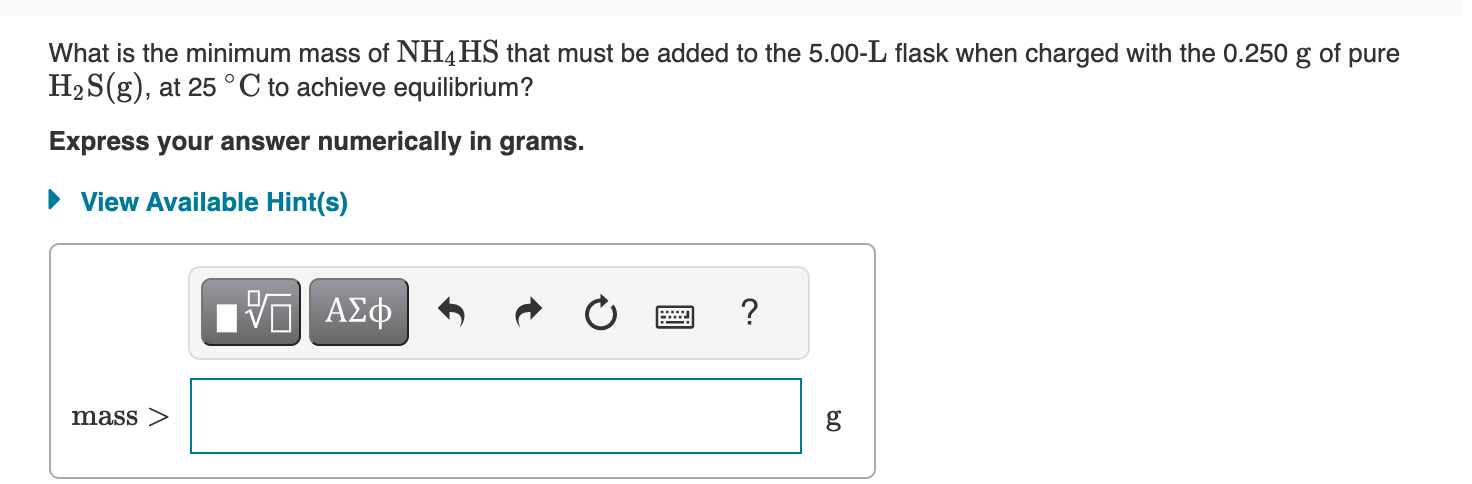
What is the initial pressure of H2S(g) in the flask? Express your answer numerically in atmospheres. An empty 5.00-L flask is charged with 0.250g of pure H2S(g), at 25C. Ammonium bisulfide, NH4HS, forms ammonia, NH3, and hydrogen sulfide, H2S, through the reaction - Part A NH4HS(s)NH3(g)+H2S(g) This reaction has a Kp value of 0.120 at 2C. - Part B Addition of ammonium bisulfate In addition to the H2S already present in the flask, solid NH4HS is added until there is excess unreacted solid remaining. What are the partial pressures of NH3 and H2S at equilibrium, that is, what are the values of PNH3 and PH2S, respectively? Enter the partial pressure of ammonia followed by the partial pressure of hydrogen sulfide numerically in atmospheres separated by a comma. What is the mole fraction, , of H2S in the gas mixture at equilibrium? Express your answer numerically. What is the minimum mass of NH4HS that must be added to the 5.00L flask when charged with the 0.250g of pure H2S(g), at 25C to achieve equilibrium? Express your answer numerically in grams
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


