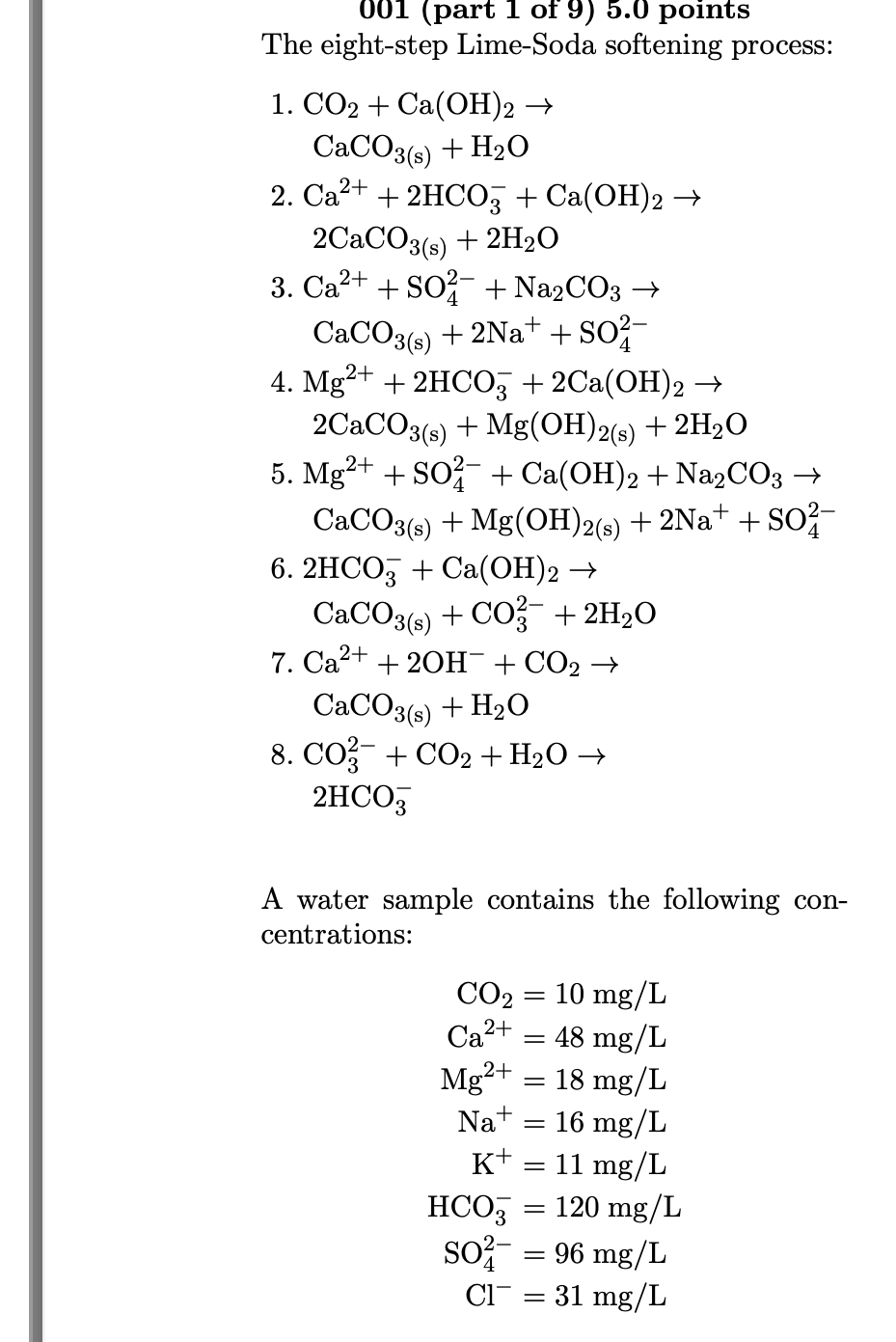
Question: Please help from questions 5 - 9 The eight-step Lime-Soda softening process: 1. CO2+Ca(OH)2 CaCO3(s)+H2O 2. Ca2++2HCO3+Ca(OH)2 2CaCO3(s)+2H2O 3. Ca2++SO42+Na2CO3 CaCO3(s)+2Na++SO42 4. Mg2++2HCO3+2Ca(OH)2 2CaCO3(s)+Mg(OH)2(s)+2H2O 5.
Please help from questions 5 - 9
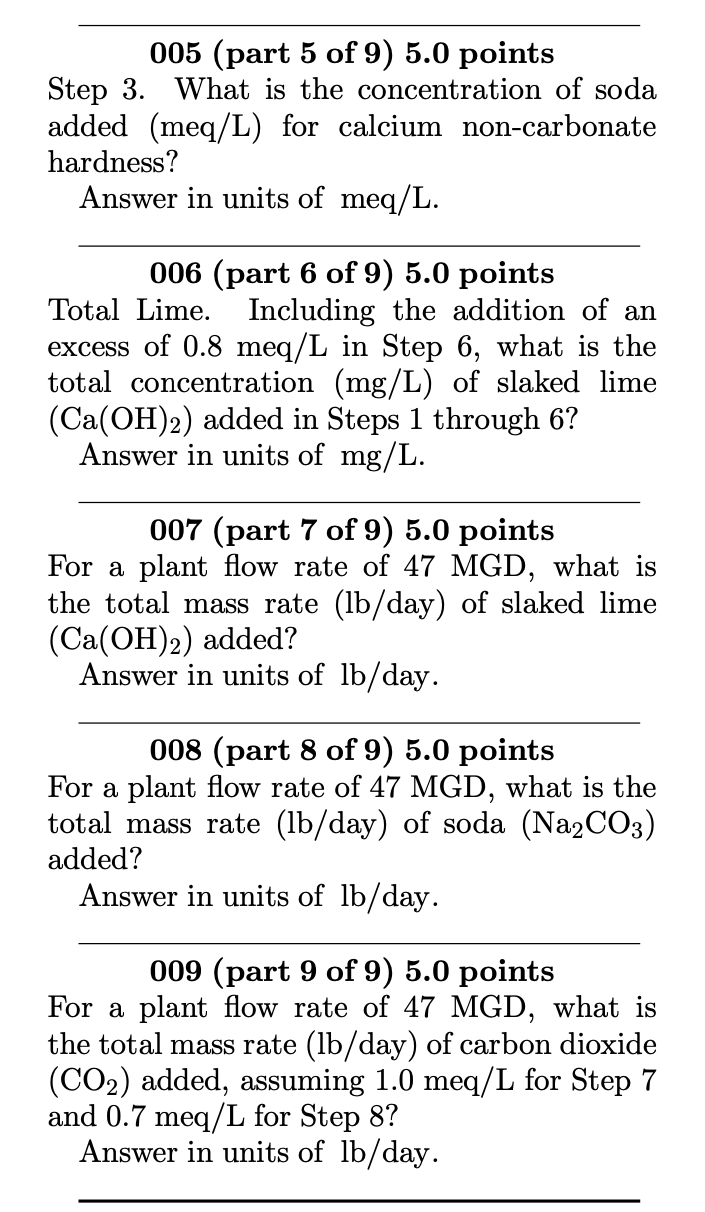
The eight-step Lime-Soda softening process: 1. CO2+Ca(OH)2 CaCO3(s)+H2O 2. Ca2++2HCO3+Ca(OH)2 2CaCO3(s)+2H2O 3. Ca2++SO42+Na2CO3 CaCO3(s)+2Na++SO42 4. Mg2++2HCO3+2Ca(OH)2 2CaCO3(s)+Mg(OH)2(s)+2H2O 5. Mg2++SO42+Ca(OH)2+Na2CO3 CaCO3(s)+Mg(OH)2(s)+2Na++SO42 6. 2HCO3+Ca(OH)2 CaCO3(s)+CO32+2H2O 7. Ca2++2OH+CO2 CaCO3(s)+H2O 8. CO32+CO2+H2O 2HCO3 A water sample contains the following concentrations: CO2Ca2+Mg2+Na+K+HCO3SO42Cl=10mg/L=48mg/L=18mg/L=16mg/L=11mg/L=120mg/L=96mg/L=31mg/L 005 (part 5 of 9) 5.0 points Step 3. What is the concentration of soda added (meq/L) for calcium non-carbonate hardness? Answer in units of meq/L. 006 (part 6 of 9) 5.0 points Total Lime. Including the addition of an excess of 0.8meq/L in Step 6, what is the total concentration (mg/L) of slaked lime (Ca(OH)2) added in Steps 1 through 6 ? Answer in units of mg/L. 007 (part 7 of 9) 5.0 points For a plant flow rate of 47 MGD, what is the total mass rate (lb/day) of slaked lime (Ca(OH)2) added? Answer in units of lb/ day. 008 (part 8 of 9) 5.0 points For a plant flow rate of 47MGD, what is the total mass rate (lb/ day) of soda (Na2CO3) added? Answer in units of lb/ day. 009 (part 9 of 9) 5.0 points For a plant flow rate of 47 MGD, what is the total mass rate (lb/day) of carbon dioxide (CO2) added, assuming 1.0meq/L for Step 7 and 0.7meq/L for Step 8 ? Answer in units of lb/ day
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


