Question: please help! i asked for help but im not sure where to start! please show step by step and double check the answer. thankyou lots
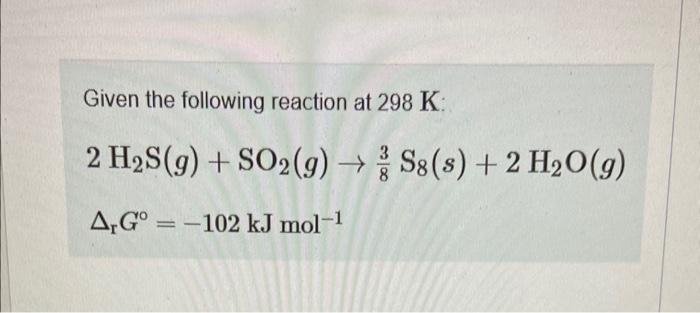
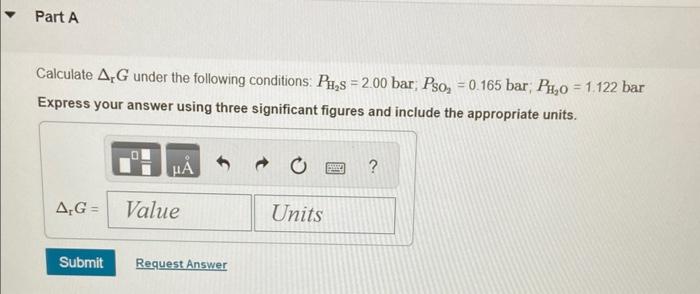

Given the following reaction at 298K : 2H2S(g)+SO2(g)83S8(s)+2H2O(g)rG=102kJmol1 Calculate rG under the following conditions: PH2S=2.00bar;PSO2=0.165 bar; PH2O=1.122 bar Express your answer using three significant figures and include the appropriate units. solve for Q then use 8 = gnot +r tin 9 Message PChatNoir
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


