Question: Please help. I can't get this one right. I have a quiz soon, so if you could please show each step of your work that
 Please help. I can't get this one right. I have a quiz soon, so if you could please show each step of your work that would be so helpful! Thank you!!
Please help. I can't get this one right. I have a quiz soon, so if you could please show each step of your work that would be so helpful! Thank you!!
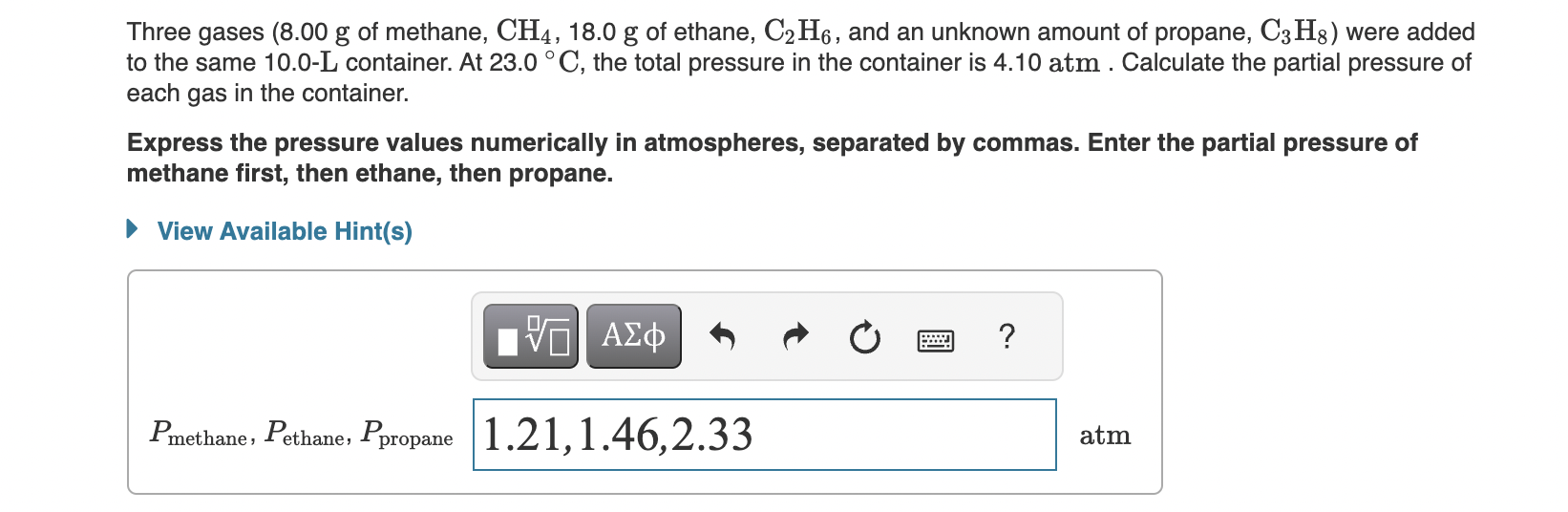
Three gases (8.00 g of methane, CH4,18.0g of ethane, C2H6, and an unknown amount of propane, C3H8 ) were added to the same 10.0-L container. At 23.0C, the total pressure in the container is 4.10atm. Calculate the partial pressure of each gas in the container. Express the pressure values numerically in atmospheres, separated by commas. Enter the partial pressure of methane first, then ethane, then propane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


