Question: please help i keep getting stuck Two beakers are placed in a small closed container at 25C. One contains 241mL of a 0.286M aqueous solution
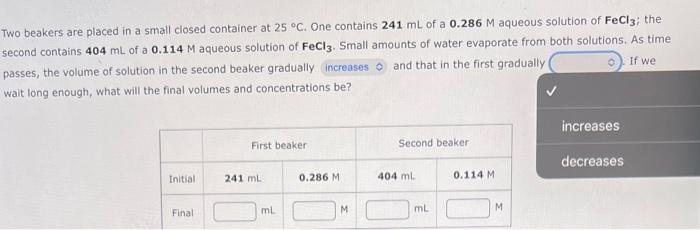
Two beakers are placed in a small closed container at 25C. One contains 241mL of a 0.286M aqueous solution of FeCl3; the second contains 404mL of a 0.114M aqueous solution of FeCl3.Small amounts of water evaporate from both solutions. As time passes, the volume of solution in the second beaker gradually and that in the first gradually If we walt long enough, what will the final volumes and concentrations be
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


