Question: Please help! I know the unknown solute is ethanol but need help solving the equations. In the lab we measured out 15mL of t-butanol and
 Please help! I know the unknown solute is ethanol but need help solving the equations. In the lab we measured out 15mL of t-butanol and added 0.250 g of the unknown solute to the t-butanol
Please help! I know the unknown solute is ethanol but need help solving the equations. In the lab we measured out 15mL of t-butanol and added 0.250 g of the unknown solute to the t-butanol
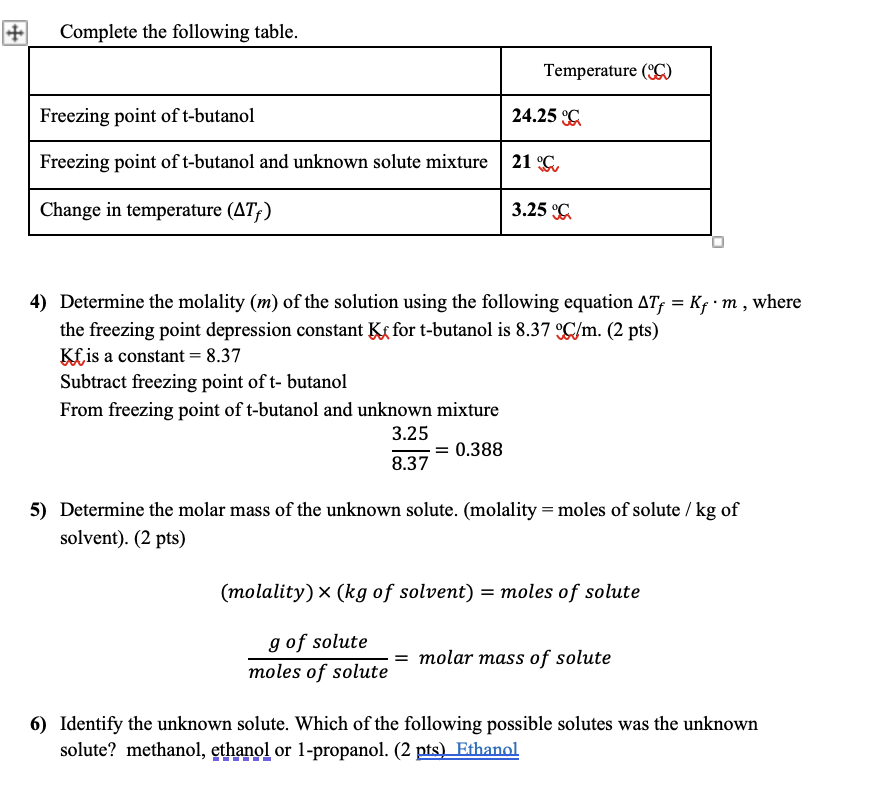
Complete the following table. 4) Determine the molality (m ) of the solution using the following equation Tf=Kfm, where the freezing point depression constant Kf for t-butanol is 8.37C/m.(2pts) Kfis a constant =8.37 Subtract freezing point of t- butanol From freezing point of t-butanol and unknown mixture 8.373.25=0.388 5) Determine the molar mass of the unknown solute. (molality = moles of solute /kg of solvent). (2 pts) (molality)(kgofsolvent)=molesofsolutemolesofsolutegofsolute=molarmassofsolute 6) Identify the unknown solute. Which of the following possible solutes was the unknown solute? methanol, ethanol or 1-propanol. ( 2nts ) Ethanol Complete the following table. 4) Determine the molality (m ) of the solution using the following equation Tf=Kfm, where the freezing point depression constant Kf for t-butanol is 8.37C/m.(2pts) Kfis a constant =8.37 Subtract freezing point of t- butanol From freezing point of t-butanol and unknown mixture 8.373.25=0.388 5) Determine the molar mass of the unknown solute. (molality = moles of solute /kg of solvent). (2 pts) (molality)(kgofsolvent)=molesofsolutemolesofsolutegofsolute=molarmassofsolute 6) Identify the unknown solute. Which of the following possible solutes was the unknown solute? methanol, ethanol or 1-propanol. ( 2nts ) Ethanol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


