Question: Please help I need the full solution fast, thats all the info I have Question 6: When 0.210 g of a gaseous compound containing only
Please help I need the full solution fast, thats all the info I have 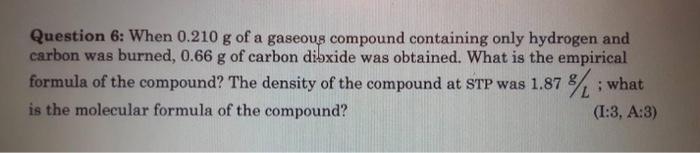
Question 6: When 0.210 g of a gaseous compound containing only hydrogen and carbon was burned, 0.66 g of carbon dibxide was obtained. What is the empirical formula of the compound? The density of the compound at STP was 1.87 % : what is the molecular formula of the compound? (I:3, A:3)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


