Question: Please help I used the equation for e and then used Qh/Th but I don't know what is wrong here 2. A Carnot engine of
Please help I used the equation for e and then used Qh/Th but I don't know what is wrong here
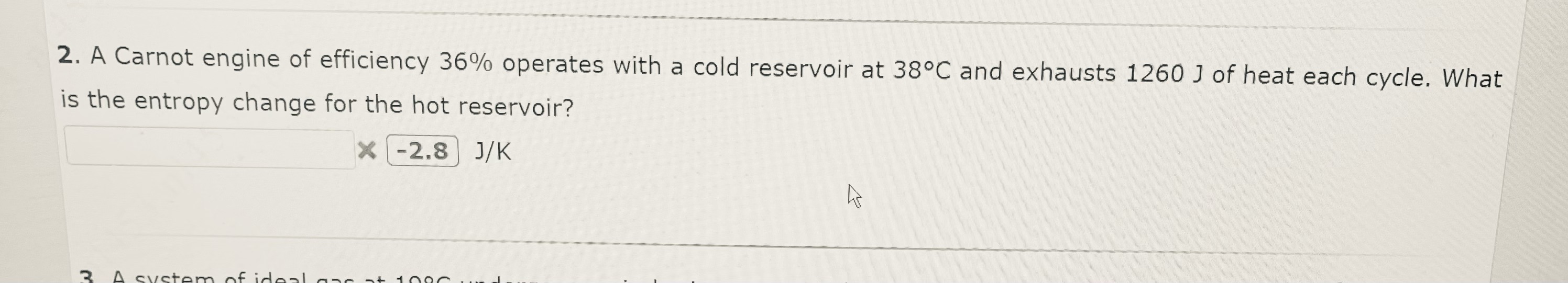
2. A Carnot engine of efficiency 36% operates with a cold reservoir at 38C and exhausts 1260 J of heat each cycle. What is the entropy change for the hot reservoir? X -2.8 J/K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


