Question: please help, im really stuck and dont know how to do it Given that the initial rate constant is 0.0150s1 at an initial temperature of
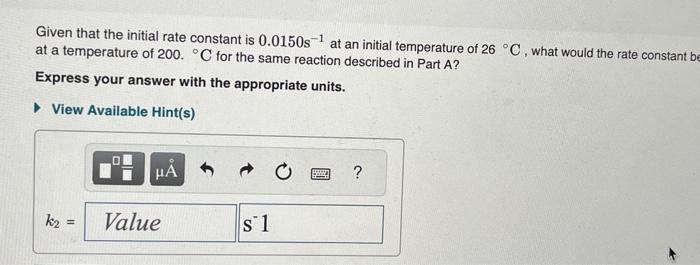

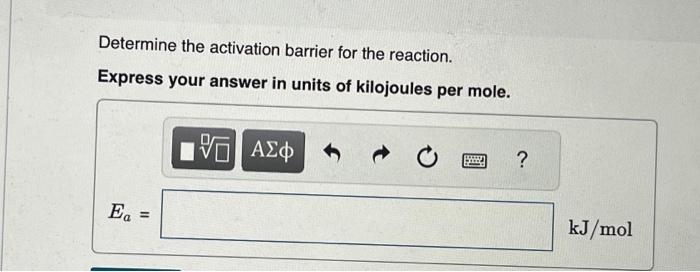
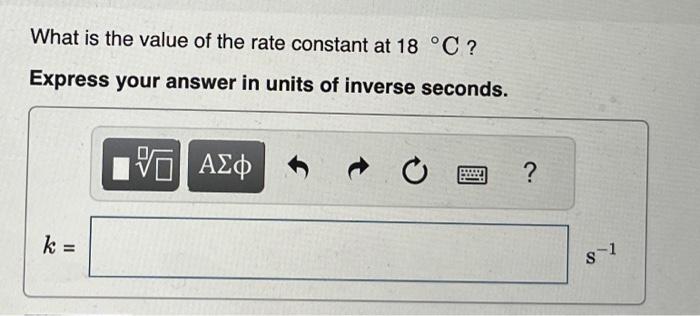
Given that the initial rate constant is 0.0150s1 at an initial temperature of 26C, what would the rate constant b at a temperature of 200 . C for the same reaction described in Part A ? Express your answer with the appropriate units. A reaction has a rate constant of 1.22104s1 at 28C and 0.228s1 at 75C. You may want to reference (Page) section 13.5 while completing this problem. Determine the activation barrier for the reaction. Express your answer in units of kilojoules per mole. What is the value of the rate constant at 18C ? Express your answer in units of inverse seconds. k=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


