Question: The heat of vaporization of ethanol (ethyl alcohol) at the normal boiling point is 38.58 kJ/mol. (a) Estimate the heat of vaporization of ethanol
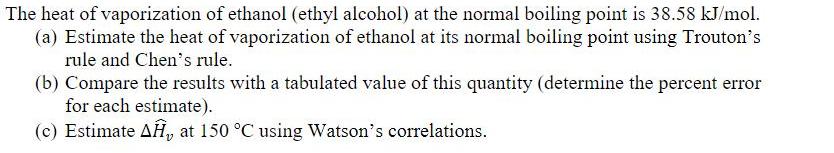
The heat of vaporization of ethanol (ethyl alcohol) at the normal boiling point is 38.58 kJ/mol. (a) Estimate the heat of vaporization of ethanol at its normal boiling point using Trouton's rule and Chen's rule. (b) Compare the results with a tabulated value of this quantity (determine the percent error for each estimate). (c) Estimate AH, at 150 C using Watson's correlations.
Step by Step Solution
3.45 Rating (165 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


