Question: Please help me 8. Balance the following equations using the half-reaction method. a) Ba(s)+Cl2Ba2++Cl b) Zn(s)+SO42+H+H2SO3(g)+Zn2++H2O c) MnO4+NO2+H2OMn2++NO3+H+ d) F+NO3+H+I2+NO2+H2O 9. For each set of
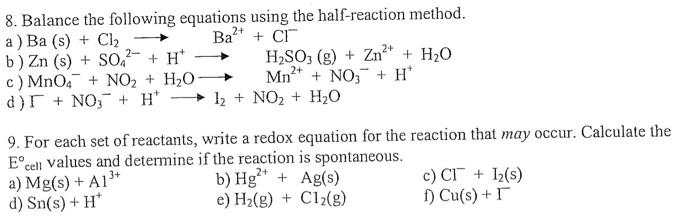
8. Balance the following equations using the half-reaction method. a) Ba(s)+Cl2Ba2++Cl b) Zn(s)+SO42+H+H2SO3(g)+Zn2++H2O c) MnO4+NO2+H2OMn2++NO3+H+ d) F+NO3+H+I2+NO2+H2O 9. For each set of reactants, write a redox equation for the reaction that may occur. Calculate the Ecell values and determine if the reaction is spontaneous. a) Mg(s)+Al3+ b) Hg2++Ag(s) c) Cl+I2 (s) d) Sn(s)+H+ e) H2(g)+Cl2(g) f) Cu(s)+I
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


