Question: please help me answer these 2 questions A certain element has a crystal lattice structure with 2 atoms per unit cell. If the atoms have
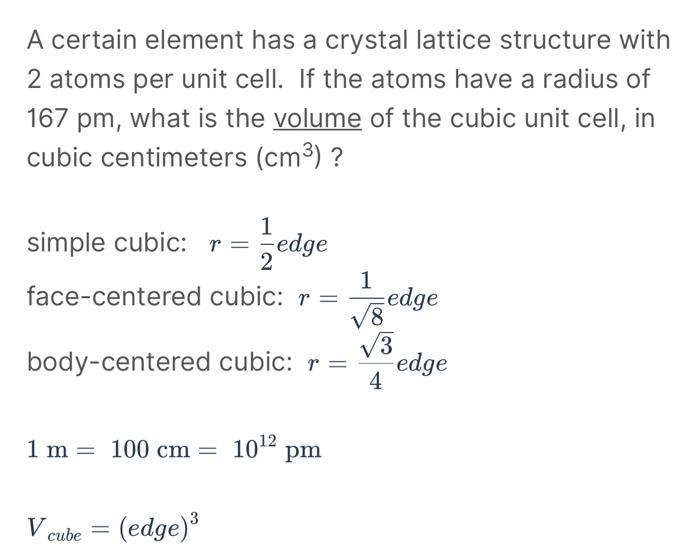
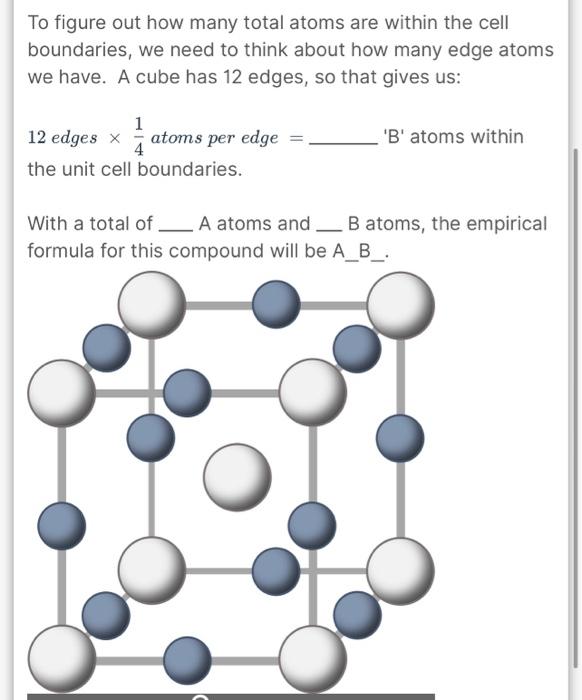
A certain element has a crystal lattice structure with 2 atoms per unit cell. If the atoms have a radius of 167pm, what is the volume of the cubic unit cell, in cubic centimeters (cm3) ? simple cubic: r=21edge face-centered cubic: r=81edge body-centered cubic: r=43edge 1m=100cm=1012pm Vcube=(edge)3 To figure out how many total atoms are within the cell boundaries, we need to think about how many edge atoms we have. A cube has 12 edges, so that gives us: 12 edges 41 atoms per edge = 'B' atoms within the unit cell boundaries. With a total of A atoms and B atoms, the empirical formula for this compound will be A_B_
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


