Question: please help me answer these 3 Qs data is on the pics below 20,22 22. Alcoholic fermentation by microorganisms involves the breakdown of glucose into
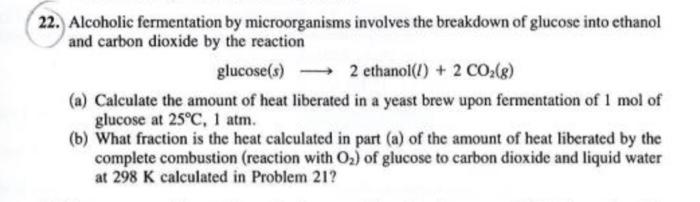


22. Alcoholic fermentation by microorganisms involves the breakdown of glucose into ethanol and carbon dioxide by the reaction glucose(s) 2 ethanol(l) + 2 CO2(8) (a) Calculate the amount of heat liberated in a yeast brew upon fermentation of 1 mol of glucose at 25C, 1 atm. (b) What fraction is the heat calculated in part (a) of the amount of heat liberated by the complete combustion (reaction with O2) of glucose to carbon dioxide and liquid water at 298 K calculated in Problem 21? 20. One mole of liquid H20 at 15C is mixed with 1 mol of liquid H,0 at 65C. The pressure is kept constant and no heat is allowed to leave the system. The process is adiabatic. Calculate the final temperature for the system. 20. One mole of liquid H20 at 15C is mixed with 1 mol of liquid H20 at 65C. The pressure is kept constant and no heat is allowed to leave the system. The process is adiabatic. Calculate the final temperature for the system. 21. A reaction that is representative of those in the glycolytic pathway is the catabolism of glucose by complete oxidation to carbon dioxide and water: C.H12Oo(s) + 6 02(8) 6 CO2(g) + 6 H20(1) Calculate AH% for the glucose oxidation. 22. Alcoholic fermentation by microorganisms involves the breakdown of glucose into ethanol and carbon dioxide by the reaction glucose(s) 2 ethanol(l) + 2 CO2(g) ) g (a) Calculate the amount of heat liberated in a yeast brew upon fermentation of 1 mol of glucose at 25C, 1 atm. (b) What fraction is the heat calculated in part (a) of the amount of heat liberated by the complete combustion (reaction with O2) of glucose to carbon dioxide and liquid water at 298 K calculated in Problem 21
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


