Question: Please help me answering this step by step, thanks!!! :) Only Excel 1. Use the Web Plot Digitizer to obtain the conversion data vs time
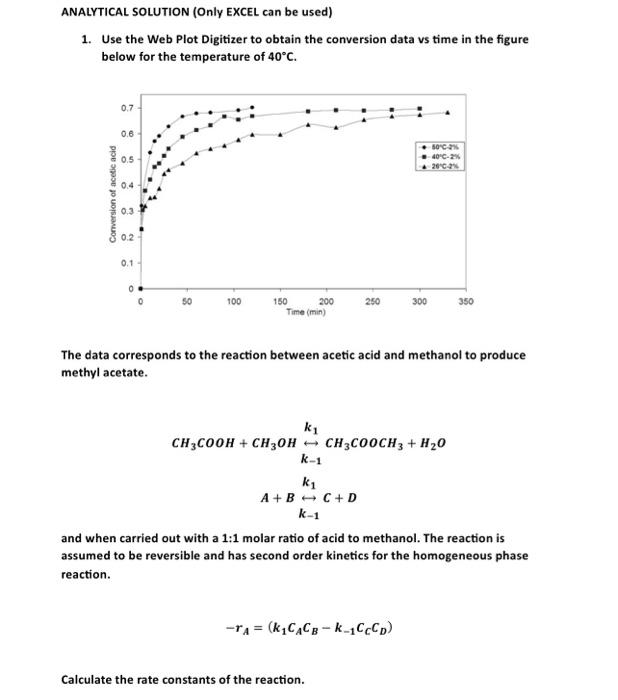
1. Use the Web Plot Digitizer to obtain the conversion data vs time in the figure below for the temperature of 40C. The data corresponds to the reaction between acetic acid and methanol to produce methyl acetate. CH3COOH+CH3OHCH3COOCH3+H2Ok1k1A+Bk1C+D and when carried out with a 1:1 molar ratio of acid to methanol. The reaction is assumed to be reversible and has second order kinetics for the homogeneous phase reaction. rA=(k1CACBk1CCCD) Calculate the rate constants of the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


