Question: Please help me answering this step by step, thanks!!! :) Only EXCEL can be used ANALYTICAL SOLUTION (Only EXCEL can be used) Eldib and Albright
Only EXCEL can be used

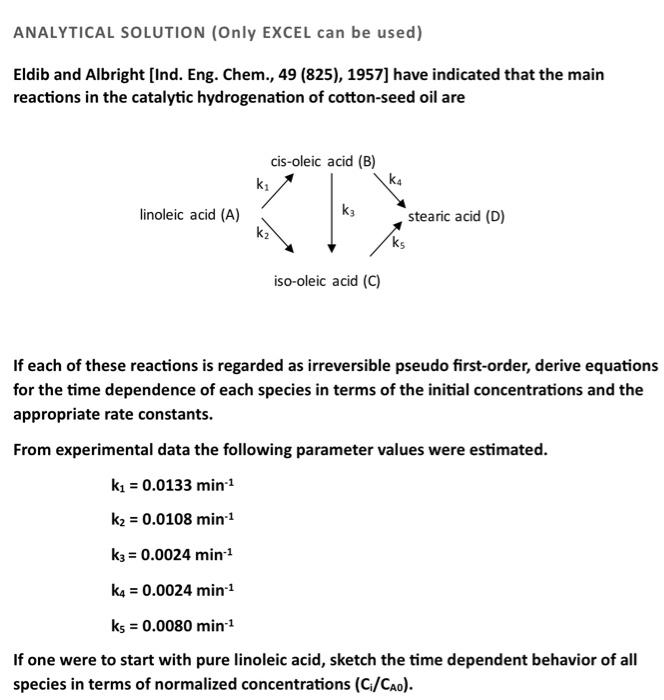
ANALYTICAL SOLUTION (Only EXCEL can be used) Eldib and Albright [Ind. Eng. Chem., 49 (825), 1957] have indicated that the main reactions in the catalytic hydrogenation of cotton-seed oil are If each of these reactions is regarded as irreversible pseudo first-order, derive equations for the time dependence of each species in terms of the initial concentrations and the appropriate rate constants. From experimental data the following parameter values were estimated. k1=0.0133min1k2=0.0108min1k3=0.0024min1k4=0.0024min1k5=0.0080min1 If one were to start with pure linoleic acid, sketch the time dependent behavior of all species in terms of normalized concentrations (Ci/CA00). ANALYTICAL SOLUTION (Only EXCEL can be used) Eldib and Albright [Ind. Eng. Chem., 49 (825), 1957] have indicated that the main reactions in the catalytic hydrogenation of cotton-seed oil are If each of these reactions is regarded as irreversible pseudo first-order, derive equation: for the time dependence of each species in terms of the initial concentrations and the appropriate rate constants. From experimental data the following parameter values were estimated. k1=0.0133min1k2=0.0108min1k3=0.0024min1k4=0.0024min1k5=0.0080min1 If one were to start with pure linoleic acid, sketch the time dependent behavior of all species in terms of normalized concentrations (Ci/CA0). ANALYTICAL SOLUTION (Only EXCEL can be used) Eldib and Albright [Ind. Eng. Chem., 49 (825), 1957] have indicated that the main reactions in the catalytic hydrogenation of cotton-seed oil are If each of these reactions is regarded as irreversible pseudo first-order, derive equations for the time dependence of each species in terms of the initial concentrations and the appropriate rate constants. From experimental data the following parameter values were estimated. k1=0.0133min1k2=0.0108min1k3=0.0024min1k4=0.0024min1k5=0.0080min1 If one were to start with pure linoleic acid, sketch the time dependent behavior of all species in terms of normalized concentrations (Ci/CA00). ANALYTICAL SOLUTION (Only EXCEL can be used) Eldib and Albright [Ind. Eng. Chem., 49 (825), 1957] have indicated that the main reactions in the catalytic hydrogenation of cotton-seed oil are If each of these reactions is regarded as irreversible pseudo first-order, derive equation: for the time dependence of each species in terms of the initial concentrations and the appropriate rate constants. From experimental data the following parameter values were estimated. k1=0.0133min1k2=0.0108min1k3=0.0024min1k4=0.0024min1k5=0.0080min1 If one were to start with pure linoleic acid, sketch the time dependent behavior of all species in terms of normalized concentrations (Ci/CA0)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


